
Introduction: Did you know that sand can be converted into a mixture of gases that spontaneously ignites in air? The procedures involved are relatively simple to perform, spectacular to observe, and relate to a rich assortment of chemical principles.
To start the process, sand and granular magnesium (I use 40-80 mesh) are combined and heated in a test tube. This results in the reduction of silicon dioxide in sand by magnesium to form magnesium oxide and elemental silicon:1,2
SiO2(s) + 2 Mg→ 2 MgO(s) + Si(s) Equation 1
You can watch a quick synopsis of this extraction of elemental silicon from sand in the video below (Video 1):
Video 1: Getting silicon out of sand, pchemstud on TikTok. Dec 9, 2021
In conjunction with the above process, magnesium silicide (Mg2Si) is formed due to the following reactions:1-3
SiO2(s) + 4 Mg(s)→ Mg2Si(s) + 2 MgO(s) Equation 2
Si(s) + 2 Mg(s)→ Mg2Si(s) Equation 3
Once the test tube has cooled, dilute HCl is added to the products. The acid reacts with Mg2Si to produce a variety of silicon-based gases that are analogous to the carbon-based alkanes:4-5
Mg2Si(s) + 4 HCl(aq)→ SiH4(g) + 2 MgCl2(aq) Equation 4
2 Mg2Si(s) + 8 HCl(aq) → Si2H6(g) + 4 MgCl2(aq) + H2(g) Equation 5
3 Mg2Si(s) + 12 HCl(aq)→ Si3H8(g) + 6 MgCl2(aq) + 2 H2(g) Equation 6
4 Mg2Si(s) + 16 HCl(aq) → Si4H10(g) + 8 MgCl2(aq) + 3 H2(g) Equation 7
This group of gases is collectively termed the silanes. In particular, SiH4 is called silane, Si2H6 disilane, Si3H8 trisilane, and Si4H10 is called tetrasilane. The silanes formed spontaneously ignite on exposure to oxygen in the air, and this produces a lot of sparks:
SiH4(g) + 2 O2(g)→ SiO2(s) + 2 H2O(l) Equation 8
2 Si2H6(g) + 7 O2(g) → 4 SiO2(s) + 6 H2O(l) Equation 9
Si3H8(g) + 5 O2(g) → 3 SiO2(s) + 4 H2O(l) Equation 10
2 Si4H10(g) + 13 O2(g) → 8 SiO2(s) + 10 H2O(l) Equation 11
Note that these spark-forming reactions are analogous to the combustion of alkanes.5 However, alkane combustion generally requires a source of ignition under normal temperature and pressures:
CH4(g) + 2 O2(g) → CO2(s) + 2 H2O(l) Equation 12
2 C2H6(g) + 7 O2(g) → 4 CO2(s) + 6 H2O(l) Equation 13
C3H8(g) + 5 O2(g) → 3 CO2(s) + 4 H2O(l) Equation 14
2 C4H10(g) + 13 O2(g) → 8 CO2(s) + 10 H2O(l) Equation 15
The video below (Video 2) outlines the various experiments involved in generating flammable gases from sand. These processes feature the chemical reactions that occur when a mixture of sand and magnesium is heated (Equations 1-3), when acid is added to magnesium silicide (Equations 4-7) and the combustion of silanes (Equations 8-11).
Video 2: Converting Sand Into Flammable Gas, Tommy Technetium YouTube Channel. Dec 30, 2021
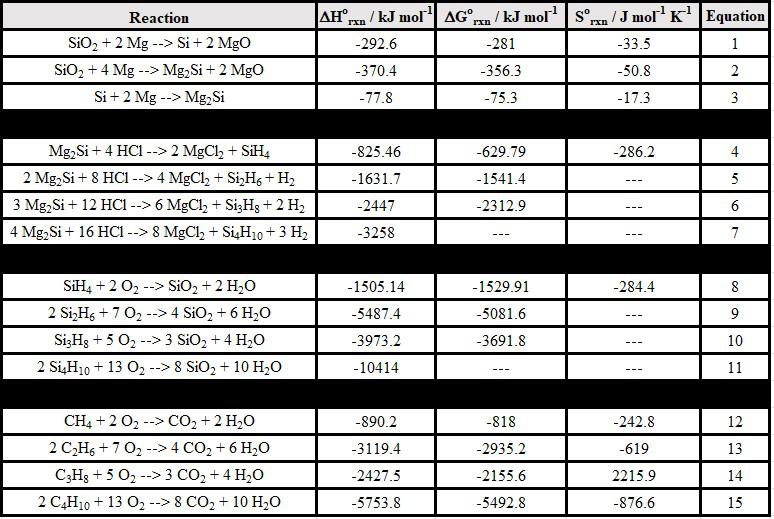
Discussion: These experiments connect to a variety of chemical topics. Standard enthalpies of formation (Table 1) may be used to calculate the standard enthalpies of all reactions involved. Standard Gibbs energies and standard entropies of reactions may also be calculated. The standard enthalpies, Gibbs energies, and entropies of all reactions listed herein are tabulated in the Appendix.
Table 1: Selected thermodynamic values6-7
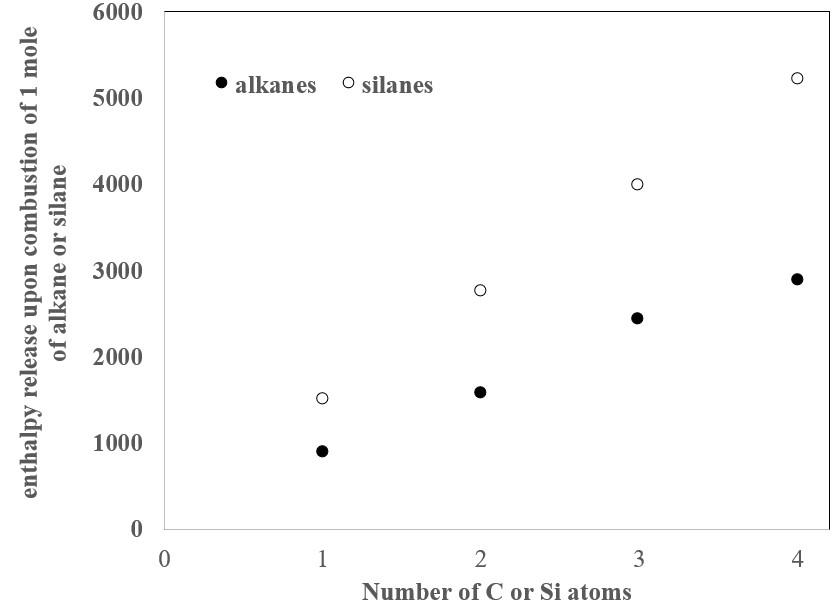
It is interesting to compare the enthalpies of combustion (Figure 1) of each silane (Equations 8-11) to the enthalpy of combustion of its corresponding alkane (Equations 12-15):
Figure 1: Enthalpy released upon combustion of the silanes and alkanes.
The fact that the silanes release more energy on combustion than the alkanes indicates that alkanes are more stable than silanes in the presence of oxygen. This fact harmonizes well with the observation that the silanes spontaneously combust in air, while the alkanes require a source of ignition to combust. Furthermore, the stability of the alkanes as compared to the silanes provides insight into the observation that long-chained molecules comprised of carbon, but not silicon, are ubiquitous. This latter observation has clear implications for why carbon-based molecules rather than silicon-based molecules provide the molecular foundation for life.
These ideas can be expanded upon further by comparing the average bond enthalpies of various chemical bonds that contain carbon and silicon (Table 2). Notice that on average it requires 141 kJ mol-1 more energy to break the carbon-carbon bond as compared to the silicon-silicon single bond. Similarly, it takes an average of 90 kJ mol-1 more energy to break the carbon-hydrogen bond as compared to the silicon-hydrogen bond. The alkanes are thus more resistant to chemical bond breaking – and therefore more resistant to chemical change – than the silanes. Further discussion on the stability of carbon-chained molecules vs. silicon-chained molecules may be found in reference 8.
Table 2: Average bond enthalpies.8,9
Conclusion: The process of converting sand into flammable silanes is intensely interesting to observe, and it entails a rich assortment of chemical topics. The array of reactions (Equations 1-11) related to the process provide a platform to discuss concepts in chemical thermodynamics and chemical bonding. Comparing the thermodynamics of silane combustion to alkane combustion allows for a discussion of the ability of carbon to form stable, long-chained molecules. Given the wide array of chemical reactions relevant to this experiment, there are certainly other chemical topics that could be discussed. I’d love to hear what chemical subjects come to your mind as you try out these experiments. I look forward to hearing your thoughts in the comments.
Happy experimenting!
Appendix: Standard enthalpies, Gibbs energies, and entropies of reactions outlined in Equations 1-15.
References:
- Borshchev, D’yachenko, Kiselev, and Kraidenko, Russian Journal of Applied Chemistry, 2013, 86 (4), 493−497.
- Favors, Wang, Hosseini Bay, Mutlu, Ahmed, Liu, Ozkan, and Ozkan, Scientific Reports, 2014, 4, 5623.
- Takamori, Osawa, Kimura, Liu. and Mukai, Materials Transactions, 2008, 49, (5) (2008), 1089-1092.
- Johnnson and Isenberg, J. Am. Chem. Soc., 1935, 57, 1349-1353.
- Johnnson, J. Chem. Educ., 1934, 11 (4), 256.
- Dean, Lange’s Handbook of Chemistry, 12th ed.; McGraw-Hill: New York, New York, 1979; pp. 9-4 - 9-94.
- Sax and Kalcher, J. Phys. Chem., 1991, 95 (4) 1768-1783.
- Smith, J. Chem. Educ., 1988, 65 (5), 414-415.
- Kotz, Treichel, and Weaver Chemistry and Chemical Reactivity, 6th ed.; Thomson Learning: Belmont, CA, 2006, p. 422.
Safety
Safety: Video Demonstration
Safety: Video Demonstration
Demonstration videos presented here are not meant as tools to teach chemical demonstration techniques. They are meant as a tool for classroom use. The demonstrations may present safety hazards or show phenomena that are difficult for an entire class to observe in a live demonstration.
Those performing the demonstrations shown in this video have been trained and adhere to best safety practices.
Anyone thinking about performing a chemistry demonstration should first read and then adhere to the ACS Safety Guidelines for Chemical Demonstrations (2016) These guidelines are also available at ChemEd X.
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Matter and its Interactions help students formulate an answer to the question, “How can one explain the structure, properties, and interactions of matter?” The PS1 Disciplinary Core Idea from the NRC Framework is broken down into three subideas: the structure and properties of matter, chemical reactions, and nuclear processes. Students are expected to develop understanding of the substructure of atoms and to provide more mechanistic explanations of the properties of substances. Chemical reactions, including rates of reactions and energy changes, can be understood by students at this level in terms of the collisions of molecules and the rearrangements of atoms. Students are able to use the periodic table as a tool to explain and predict the properties of elements. Using this expanded knowledge of chemical reactions, students are able to explain important biological and geophysical phenomena. Phenomena involving nuclei are also important to understand, as they explain the formation and abundance of the elements, radioactivity, the release of energy from the sun and other stars, and the generation of nuclear power. Students are also able to apply an understanding of the process of optimization in engineering design to chemical reaction systems. The crosscutting concepts of patterns, energy and matter, and stability and change are called out as organizing concepts for these disciplinary core ideas. In the PS1 performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and conducting investigations, using mathematical thinking, and constructing explanations and designing solutions; and to use these practices to demonstrate understanding of the core ideas.
*More information about this category of NGSS can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions.
"Matter and its Interactions help students formulate an answer to the question, “How can one explain the structure, properties, and interactions of matter?” The PS1 Disciplinary Core Idea from the NRC Framework is broken down into three subideas: the structure and properties of matter, chemical reactions, and nuclear processes. Students are expected to develop understanding of the substructure of atoms and to provide more mechanistic explanations of the properties of substances. Chemical reactions, including rates of reactions and energy changes, can be understood by students at this level in terms of the collisions of molecules and the rearrangements of atoms. Students are able to use the periodic table as a tool to explain and predict the properties of elements. Using this expanded knowledge of chemical reactions, students are able to explain important biological and geophysical phenomena. Phenomena involving nuclei are also important to understand, as they explain the formation and abundance of the elements, radioactivity, the release of energy from the sun and other stars, and the generation of nuclear power. Students are also able to apply an understanding of the process of optimization in engineering design to chemical reaction systems. The crosscutting concepts of patterns, energy and matter, and stability and change are called out as organizing concepts for these disciplinary core ideas. In the PS1 performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and conducting investigations, using mathematical thinking, and constructing explanations and designing solutions; and to use these practices to demonstrate understanding of the core ideas."
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.
Students who demonstrate understanding can develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
Assessment does not include calculating the total bond energy changes during a chemical reaction from the bond energies of reactants and products.
Emphasis is on the idea that a chemical reaction is a system that affects the energy change. Examples of models could include molecular-level drawings and diagrams of reactions, graphs showing the relative energies of reactants and products, and representations showing energy is conserved.
Energy help students formulate an answer to the question, “How is energy transferred and conserved?” The Core Idea expressed in the Framework for PS3 is broken down into four sub-core ideas: Definitions of Energy, Conservation of Energy and Energy Transfer, the Relationship between Energy and Forces, and Energy in Chemical Process and Everyday Life. Energy is understood as quantitative property of a system that depends on the motion and interactions of matter and radiation within that system, and the total change of energy in any system is always equal to the total energy transferred into or out of the system. Students develop an understanding that energy at both the macroscopic and the atomic scale can be accounted for as either motions of particles or energy associated with the configuration (relative positions) of particles. In some cases, the energy associated with the configuration of particles can be thought of as stored in fields. Students also demonstrate their understanding of engineering principles when they design, build, and refine devices associated with the conversion of energy. The crosscutting concepts of cause and effect; systems and system models; energy and matter; and the influence of science, engineering, and technology on society and the natural world are further developed in the performance expectations associated with PS3. In these performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and carry out investigations, using computational thinking and designing solutions; and to use these practices to demonstrate understanding of the core ideas.*
*More information about all DCI for HS-PS3 can be found at https://www.nextgenscience.org/topic-arrangement/hsenergy.
Energy help students formulate an answer to the question, “How is energy transferred and conserved?” The Core Idea expressed in the Framework for PS3 is broken down into four sub-core ideas: Definitions of Energy, Conservation of Energy and Energy Transfer, the Relationship between Energy and Forces, and Energy in Chemical Process and Everyday Life. Energy is understood as quantitative property of a system that depends on the motion and interactions of matter and radiation within that system, and the total change of energy in any system is always equal to the total energy transferred into or out of the system. Students develop an understanding that energy at both the macroscopic and the atomic scale can be accounted for as either motions of particles or energy associated with the configuration (relative positions) of particles. In some cases, the energy associated with the configuration of particles can be thought of as stored in fields. Students also demonstrate their understanding of engineering principles when they design, build, and refine devices associated with the conversion of energy. The crosscutting concepts of cause and effect; systems and system models; energy and matter; and the influence of science, engineering, and technology on society and the natural world are further developed in the performance expectations associated with PS3. In these performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and carry out investigations, using computational thinking and designing solutions; and to use these practices to demonstrate understanding of the core ideas



All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 2
Comparing stability
Hi Tom,
It is a very interesting set of reactions and the procedures are so simple but the effects are spectacular. I think typical high school students will very much enjoy this experiment.
I have one question in regard to your discussion. As you mention both C-C and C-H bonds are more stable than Si-Si and Si-H bonds, should we say alkane is more stable than silane? The fact silane spontaneously combusts in the air but alkane needs some initial ignition. I am confused about your statement "The fact that the silanes release more energy on combustion than the alkanes indicates that silanes are more stable than alkanes in the presence of oxygen."
Yu-Sung
Thanks for pointing out my error!
Yu-Sung:
You are indeed correct: The alkanes are more stable than the silanes. Thank you so much for catching my mistake. I will fix the erroneous sentence you cited to reflect this.
With appreciation,
Tom