
The inaugural ChemEdX Conference: Chemistry Instruction for the Next Generation is wrapping up this week. The first session was based on a Journal of Chemical Education article, “Evidence for the Effectiveness of Inquiry-Based, Particulate-Level Instruction on Conceptions of the Particulate Nature of Matter”, authored by Chad Bridle (Grandville HS, Grandville, MI) and Ellen Yezierski (Miami University, Oxford, OH). (This is an ACS Author’s Choice article, meaning that you can access it for free without a subscription to JCE.) This was great timing for me because my freshmen classes were just beginning to learn about physical properties and changes and I have used the “Change You Can Believe In” inquiry-based activity for several semesters now. In this blog post, I want to share how I incorporate the Target Inquiry activity for the first part of the unit.
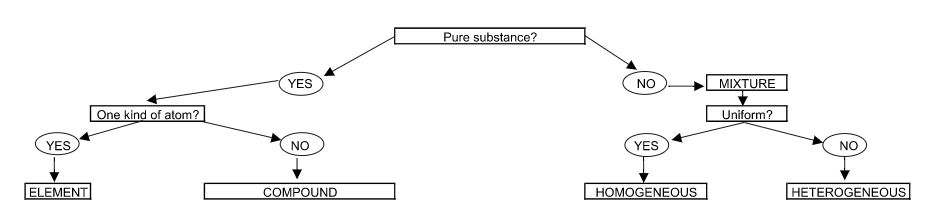
On Day 1, I write out a flowchart for students to emulate to help define pure and impure substances; element, compounds, and mixtures; and homogeneous and heterogeneous mixtures (see figure 1). It looks similar to the following and I include definitions along with it.
Figure 1: Flowchart to identify types of substances
Next, I introduce solutions and describe the difference between solute and solvent. I provide examples of solutions with varying states of matter (dependent on solvent state). My favorite example is a wet diaper since I have two daughters under age 2 at home. Kids might be grossed out until I get my “Instant Snow Polymer” out that I purchased at an NSTA conference years ago. “Instant Snow Polymer” is comprised of sodium polyacrylate (active ingredient in diapers) and it can expand to 40 times its original volume when water is added. Students love this demo, especially when they volunteer!
By Day 2, we begin a discussion of the states of matter and students work through the pre-lab portion of “Change You Can Believe In.” We discuss how one should approach particulate models and talk about their significance.
Day 3 and 4 consist of the “Change You Can Believe In” activity where students are tasked with understanding, describing, and demonstrating the differences between physical and chemical changes in the form of particulate level models. See figure 2 for some examples of the particulate models students look at:
Figure 2: Sample cards from the "Change You Can Believe In" activity
We wrap up Part 1 of the unit by learning about gas laws (qualitative aspect) and the difference between heat and temperature (using the “Incredible Ice Melting Blocks” demo). Students often believe that ice will melt faster on the warmer block or at equal rates. Last semester and this semester I decided to give students an assessment halfway through the unit and then cover phase changes separately. In doing so this semester, I contacted Chad Bridle for example assessment questions that included more particulate models. I had been reflecting and realized that even though we were using them in class, I wasn’t assessing students with the models; this had to change because I have found that using particulate models within the assessments helps me better evaluate my student's conceptual understanding.
NGSS
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds. Use a model to predict the relationships between systems or between components of a system.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments.





All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 2
Thank you
I use this activity regularly but I like your method of design better! Will definately try it at the beginning of the year. Thanks for sharing and thanks for participating in the conference.
You bet, Chad! Thanks for
You bet, Chad! Thanks for reading. I'm glad I could provide some useful information.