
I recently had the opportunity to attend a conference of the Associated Chemistry Teachers of Texas (ACT2). I had great time interacting with and learning from a whole bunch of wonderful chemical educators from the great state of Texas. One of the most interesting things I learned was in reference to the classic “whoosh bottle” experiment, which is powered by the combustion of isopropyl alcohol:
2 C3H7OH(l) + 9 O2(g) → 6 CO2(g) + 8 H2O(g) Equation 1
Interestingly, the reaction in this experiment yields different results when conducted at different temperatures (Video 1).
Video 1: Effect of temperature on a combustion reaction, pchemstud on TikTok. June 20, 2022.
That’s quite a significant difference! When I shared this video online, I had a few people comment that the difference observed might not be due to a faster reaction rate at the higher temperature. Instead, it was argued that the difference was because more alcohol vapor is contained in the warm bottle as compared to the cold one, on account of the higher vapor pressure of alcohol at higher temperatures. We can see this quantitatively by first noting the vapor pressures of isopropyl alcohol at 5.0oC (12 mmHg = 0.016 atm) and 32 oC and 20oC (32 mm Hg = 0.042 atm).1 Because the volume of the bottle is known (5 gallons = 19 L), we can then use the idea gas law (as n = PV/RT) to calculate the moles of isopropyl alcohol present in each bottle:
That’s about 2.5 times more alcohol vapor in the warm bottle over the cool one. Because reaction rates increase with higher concentration of reactants, perhaps the effect observed really is due to the presence of more fuel in the warm bottle.
Or is it…?
I decided to look into this a bit further, attempting to use the Arrhenius Equation to compare the kinetic rates of the reaction at the different temperatures:
Where k is the rate constant for the reaction, A is the pre-exponential factor, Ea is the activation energy for the reaction, T is temperature, and R = 8.314 J mol-1 K-1. The ratio of the values of the rate constants at 20oC (293 K) and 5oC (278 K) would therefore be:
Substitution of 125 kJ mol-1 as an estimate for the activation energy of the combustion of isopropanol2-4 into Equation 3 yields:
Therefore, this analysis suggests combustion in the warm bottle should proceed about 16 times faster than in the cold bottle.
So which is it?
Well, 16 (kinetic temperature effect) is over 5 times bigger than 2.5 (amount of fuel effect), so perhaps these analyses should lead me to conclude that combustion in the hot “whoosh bottle” goes faster because of the effect of temperature on reaction rates – not from the presence of more fuel in the hot bottle as compared to the cool one. But honestly, I’m not sure. I’m not entirely comfortable with my estimation of the activation energy of isopropanol combustion used in the Arrhenius analysis. I’m also not sure that the Arrhenius analysis I did is entirely appropriate. Furthermore, what I am confident about is that multiple types of combustion took place in the warm bottle but not in the cool bottle. I say this because I saw soot had formed on the outside of the warm bottle, but not the cool one after the reaction subsided (look for this carefully in Video 1). The formation of soot indicates incomplete combustion, in which carbon monoxide (Equation 4) and soot (Equation 5) are formed:
C3H7OH(l) + 3 O2(g) → 3 CO(g) + 4 H2O(g) Equation 4
2 C3H7OH(l) + 3 O2(g) → 6 C (s, soot) + 8 H2O(g) Equation 5
If multiple reactions occurred in the warm bottle but only complete combustion in the cool bottle, this complicates matters further. In the end, if I had to guess, I’d venture that both effects play a role.
But I’d like to know what you think. Why does the “whoosh bottle” go faster at higher temperatures? Is it simply because of the effect of temperature on reaction kinetics? Or is it because more alcohol vapor is present in the warmer bottle due to the higher vapor pressure of alcohol at increased temperature? Might both impact the reaction rate? What thoughts or criticisms do you have on my analysis presented here? Do you have any suggestions for experiments I might try to test which of these two possibilities better describes the observations? I look forward to hearing from you, and to carrying out more experiments on the effect of temperature on the “whoosh bottle.”
Happy Experimenting!
Acknowledgement:
Thanks to Dr. Bob Shelton from Texas A&M University in San Antonio for showing me this demonstration.
References:
- Parks, G. S.; Barton, B. J. Am. Chem. Soc. 1928, 50, 24-26.
- Vandenabeele, H.; Corbeels, R.; van Tiggelen, A. Combustion and Flame, 1960, 4, 253-260.
- Frassoldati, A.; Cuoci, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; , Seiser, R.; Seshadri, K. Combustion and Flame, 1960, 4, 253-260.
- I could not find a literature value for experimentally measured values for the activation energy of the combustion of isopropanol. However, reference 3 does mention a value of less than 35 kcal mol-1 (146 kJ mol-1) based on a combination of measurements and theoretical calculation. Further, reference 2 cites 36.5 kcal mol-1 (153 kJ mol-1) as the activation energy for methane combustion. These considerations suggest 125 kJ mol-1 is a fair estimate.
Safety
Safety: Video Demonstration
Safety: Video Demonstration
Demonstration videos presented here are not meant as tools to teach chemical demonstration techniques. They are meant as a tool for classroom use. The demonstrations may present safety hazards or show phenomena that are difficult for an entire class to observe in a live demonstration.
Those performing the demonstrations shown in this video have been trained and adhere to best safety practices.
Anyone thinking about performing a chemistry demonstration should first read and then adhere to the ACS Safety Guidelines for Chemical Demonstrations (2016) These guidelines are also available at ChemEd X.
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Asking questions and defining problems in grades 9–12 builds from grades K–8 experiences and progresses to formulating, refining, and evaluating empirically testable questions and design problems using models and simulations.
Asking questions and defining problems in grades 9–12 builds from grades K–8 experiences and progresses to formulating, refining, and evaluating empirically testable questions and design problems using models and simulations.
questions that challenge the premise(s) of an argument, the interpretation of a data set, or the suitability of a design.
Scientific questions arise in a variety of ways. They can be driven by curiosity about the world (e.g., Why is the sky blue?). They can be inspired by a model’s or theory’s predictions or by attempts to extend or refine a model or theory (e.g., How does the particle model of matter explain the incompressibility of liquids?). Or they can result from the need to provide better solutions to a problem. For example, the question of why it is impossible to siphon water above a height of 32 feet led Evangelista Torricelli (17th-century inventor of the barometer) to his discoveries about the atmosphere and the identification of a vacuum.
Questions are also important in engineering. Engineers must be able to ask probing questions in order to define an engineering problem. For example, they may ask: What is the need or desire that underlies the problem? What are the criteria (specifications) for a successful solution? What are the constraints? Other questions arise when generating possible solutions: Will this solution meet the design criteria? Can two or more ideas be combined to produce a better solution?
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Matter and its Interactions help students formulate an answer to the question, “How can one explain the structure, properties, and interactions of matter?” The PS1 Disciplinary Core Idea from the NRC Framework is broken down into three subideas: the structure and properties of matter, chemical reactions, and nuclear processes. Students are expected to develop understanding of the substructure of atoms and to provide more mechanistic explanations of the properties of substances. Chemical reactions, including rates of reactions and energy changes, can be understood by students at this level in terms of the collisions of molecules and the rearrangements of atoms. Students are able to use the periodic table as a tool to explain and predict the properties of elements. Using this expanded knowledge of chemical reactions, students are able to explain important biological and geophysical phenomena. Phenomena involving nuclei are also important to understand, as they explain the formation and abundance of the elements, radioactivity, the release of energy from the sun and other stars, and the generation of nuclear power. Students are also able to apply an understanding of the process of optimization in engineering design to chemical reaction systems. The crosscutting concepts of patterns, energy and matter, and stability and change are called out as organizing concepts for these disciplinary core ideas. In the PS1 performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and conducting investigations, using mathematical thinking, and constructing explanations and designing solutions; and to use these practices to demonstrate understanding of the core ideas.
*More information about this category of NGSS can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions.
"Matter and its Interactions help students formulate an answer to the question, “How can one explain the structure, properties, and interactions of matter?” The PS1 Disciplinary Core Idea from the NRC Framework is broken down into three subideas: the structure and properties of matter, chemical reactions, and nuclear processes. Students are expected to develop understanding of the substructure of atoms and to provide more mechanistic explanations of the properties of substances. Chemical reactions, including rates of reactions and energy changes, can be understood by students at this level in terms of the collisions of molecules and the rearrangements of atoms. Students are able to use the periodic table as a tool to explain and predict the properties of elements. Using this expanded knowledge of chemical reactions, students are able to explain important biological and geophysical phenomena. Phenomena involving nuclei are also important to understand, as they explain the formation and abundance of the elements, radioactivity, the release of energy from the sun and other stars, and the generation of nuclear power. Students are also able to apply an understanding of the process of optimization in engineering design to chemical reaction systems. The crosscutting concepts of patterns, energy and matter, and stability and change are called out as organizing concepts for these disciplinary core ideas. In the PS1 performance expectations, students are expected to demonstrate proficiency in developing and using models, planning and conducting investigations, using mathematical thinking, and constructing explanations and designing solutions; and to use these practices to demonstrate understanding of the core ideas."
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.
Students who demonstrate understanding can apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
Assessment is limited to simple reactions in which there are only two reactants; evidence from temperature, concentration, and rate data; and qualitative relationships between rate and temperature.
Emphasis is on student reasoning that focuses on the number and energy of collisions between molecules.

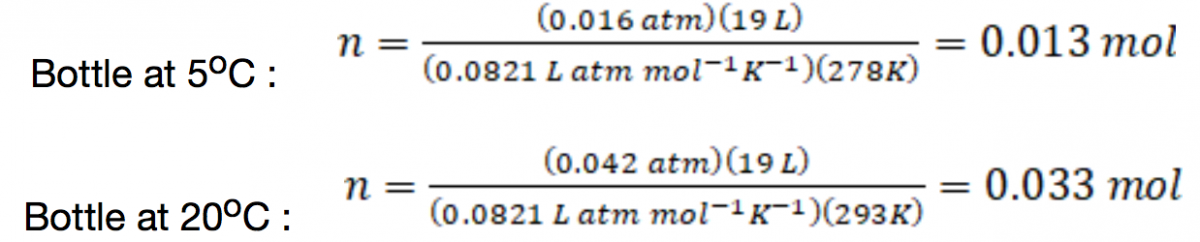


All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 3
On whether the concentration or temperature influences more.
I think that to verify this, it is enough to measure the time it takes to carry out each of the reactions, since it is very easy to calculate that time on video, even if it is approximate. I have taken the trouble to measure this time, and in the first reaction at 5ºC it takes approximately 5 s to complete and in the second at 20ºC it takes approximately 2 s, then the first is 2.5 times slower than the second or vice versa the second is 2.5 times faster, curiously exactly the largest amount of alcohol there is, this is no coincidence. If we remember the kinetics of the reactions, in the first place we have the concentration of reagents and secondly the Temperature, and studying it like this in that order is not by chance seeing what happened. It can be assumed that it is at the same concentrations where the effect of temperature is relevant, but at different concentrations the temperature hardly has any effect on the kinetics of the reaction.
Saludos.
(Sobre si influye mas la concentración o la temperatura. Creo que para comprobar esto es suficiente con medir el tiempo que tarda en realizarse cada una de las reacciones, ya que esta en video es muy fácil calcular ese tiempo, aunque sea aproximado. M ehe tomado la moletia de medir dicho tiempo, y en la primera reaccion a 5ºC tarda aproximadamente 5 s en completarse y en la segunda a 20ºC tarda aproximadamente 2 s, luego la primera es 2,5 veces mas lenta que la segunda o al revés la segunda es 2,5 veces mas rápida, curiosamente exactamente la cantidad mayor de alcohol que hay, esto no es casualidad. Si recordamos la cinética de las reacciones, en primer lugar tenemos la concetración de reactivos y en segundo lugar la Temperatura, y estudiarlo así en ese orden no es casualidad viendo lo ocurrido. Se puede suponer que es a iguales concentraciones donde el efecto de la temperatura es relevante, pero a distintas concentraciones la temperatura apenas tiene efectos sobre la cinética de la reacción.)
Nice analysis!
Hi Josefpm,
Thank you so much for taking the time to analyze how long it takes for each of these reactions to complete. What a great idea! It is indeed very interesting that the reaction happens 2.5 times more quickly at a warmer temperature than at a cooler temperature, and that this matches the estimated difference in concentration of the vapor in each container! In my opinion, this does not demonstrate that the reaction is only influenced by the differences in concentration, and that temperature has no effect on the kinetics. I would argue that the match in concentration and reaction time could indeed be coincidence. I say this because we certainly know that temperature influences the rates of reactions. Of course concentration does as well, as you rightly point out.
What do you think, Josepfpm? Am I off base? How might you provide further evidence to convince me that the difference in reaction kinetics is due only to concentration and not temperature?
I wonder what others think about this. Anyone else want to chime in?
Again, thanks so much for the discussion!
I agree but not completely ;-)
Oh, thanks for taking the time to answer, I agree with you, that does not mean that the temperature does not affect it, but I do believe, as I indicated, that at different concentrations the determining factor is the concentration and not the temperature, although the temperature has its effect, this effect is much less. That is why I suppose that at the same concentration the effect of temperature or at very high temperatures would be much more decisive. could it be coincidence? Yes, it could be, but wouldn't it be too much of a coincidence? and more when we know that the concentration in a factor that influences the speed of reaction. It would be a matter of carrying out the experiment with other concentrations and measuring the speed and seeing if that relationship is maintained or, as he says, it was coincidence. The question would not be to see if the temperature affects or not the speed of reaction, or if the concentration affects or not, it is evident that both affect; The question would be to what extent do they affect? which is more decisive? It occurs to me that you can try carrying out the HCl+NaHCO3 reaction, using the same concentration at two temperatures and measuring the reaction time, it would be approximate, for example at room temperature and inside a refrigerator or cold room... umm I'll try to do it this summer if I have time...
--------
(Oh, gracias por tomarte tiempo en contestar, coincido con usted, eso no indica que la temperatura no afecte, pero si creo, como indico, que a diferentes concentraciones el factor determinante es la concentración y no la temperatura, aunque la temperatura tenga su efecto este efecto es mucho menor. Por eso supongo que a igual concentración si sería mucho mas determinante el efecto de la temperatura o a muy altas temperaturas. ¿podría ser casualidad? Si, podría ser pero, ¿no sería demasiada casualidad? y más cuando sabemos que la concentración en un factor que influye en la velocidad de reacción. Sería cuestión de realizar el experimento con otras concentraciones y medir la velocidad y ver si se mantiene ese relación o como dice fue casualidad. La cuestión no sería ver si la temperatura afecta o no a la velocidad de reacción, o si la concentración afecta o no, es evidente que ambas afectan; la cuestión sería ¿en qué medida afectan? ¿cual es mas determinante? Se me ocurre que se puede probar a realizar la reacción del HCl+NaHCO3, usando al misma concentración a dos temperaturas y medir el tiempo, de reacción, sería aproximado, por ejemplo a temperatura ambiente y dentro de un refrigerador o cámara frigorífica... umm intentaré hacerlo este verano si tengo tiempo...)