Scrub Daddy is a cleaning supply that was invented by Aaron Krause1 and is sold as America’s Favorite Sponge.2 After being featured on ABC’s television show Shark Tank, Scrub Daddy has gone on to be Shark Tank’s most successful product, selling over 10 million products and generating $50 million in sales.3 Part of the appeal of the Scrub Daddy sponge is that it changes from soft to hard, depending upon temperature. This allows a single sponge to be transformed into a hard scrubber or soft sponge, depending upon the temperature of water into which it is placed. Check it out:
When I first learned about Scrub Daddy sponges, I immediately wondered what might be the science behind its temperature-driven, hard-to-soft transformation. My initial hunch was that chemistry (of course!) was somehow involved. Upon finding and reading the patent for Scrub Daddy, I learned that a Scrub Daddy sponge is comprised of a mixture of polymers, or long-chained molecules.1 Further, it is likely polycaprolactone1 (Figure 1) or some derivative thereof that is responsible for the hard-to-soft transition as the Scrub Daddy sponge is warmed.
Figure 1 - Structure of polycaprolactone. Several units of the monomer shown link together in a chain to form the polymer.
Polycaprolactone belongs to a class of polymers known as thermoplastics, which change in degree of stiffness as they move from cold (rigid) to hot (soft). It is interesting to note that polycaprolactone is used in a variety of biomedical devices such as drug delivery agents, sutures, and bone/cartilage fabrication.4–6
I purchased a Scrub Daddy sponge to investigate at what temperature the hard-to-soft transition occurred. To test this, I first measured the thickness of the Scrub Daddy. Next, I put some very hot tap water into a large bowl, and immersed the Scrub Daddy in the water for 30 seconds. After checking the temperature of the water, I placed the Scrub Daddy on a hard surface, placed six pounds of weight on top, and measured the thickness of the Scrub Daddy with the weights on top. I repeated this process at a variety of temperatures (Figure 2).
Figure 2 - Compression of Scrub Daddy with 6 pounds of added weight at (L to R) 60oC, 23oC, and 10oC.
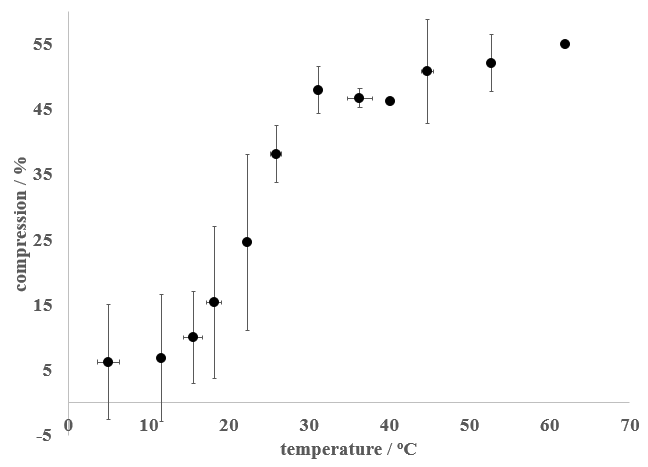
I then plotted the compression of the sponge as a function of temperature (Figure 3), with compression, C, taken as:
Where h0 is the thickness of the sponge without added weight and h is the thickness of the sponge with added weight. Thus, a high value of C corresponded to an easily deformed sponge, while a low C value corresponded to a sponge that did not easily deform. The Scrub Daddy showed a marked change in compression in the 15 – 30oC temperature range, with lower temperatures displaying less compression than higher temperatures.
Figure 3 - Effect of temperature on the compression of a Scrub Daddy sponge. The error bars represent one standard deviation (at least 3 trials were conducted for each data point displayed).
The range over which the sponge softened was substantially lower than the melting temperature of polycaprolactone of 58oC.7 This made me wonder if it was really some other polymer – and not polycaprolactone – that was responsible for the temperature dependent softening observed in the Scrub Daddy. I therefore decided to take an infrared spectrum (IR) of the Scrub Daddy sponge (Figure 4).
Figure 4 -FTIR spectrum of a Scrub Daddy sponge.
The spectrum displayed several features that were very similar to a previously reported IR spectrum of polycaprolactone.8 Therefore, a Scrub Daddy sponge almost certainly contains polycaprolactone! It is my guess that polymers other than polycaprolactone have been added to a Scrub Daddy in order to lower the temperature at which the sponge softens, and also to keep it from getting sticky at very high temperatures. My guess is consistent with statements in the Sponge Daddy patent, which reports that polymer blends are used in the sponge.1
Polycaprolactone is quite easy to obtain,9 so I purchased some to investigate its properties. You can see some of these investigations in the video below.
I plan on using some of the experiments reported here in my classes. If you do the same, let me know in the comments how things work out for you. Also, please consider commenting if you have suggestions on how to extend any of these experiments. I’d also like to hear from you if you think any of my interpretations of what is going on in these experiments are off base. Happy experimenting!
References:
- https://www.google.com/patents/US20140075699
- https://scrubdaddy.com/
- http://www.businessinsider.com/scrub-daddy-shark-tank-success-story-2017-3
- http://pubs.acs.org/doi/10.1021/ed2004615
- http://pubs.acs.org/doi/10.1021/acs.jchemed.6b00835
- http://artelon.com/pdf/WoodruffMAProgrPolymerSciInPress2010.pdf
- https://www.researchgate.net/publication/290808994_Polymer_Data_Handbook
- https://www.researchgate.net/publication/299537578_Synthesis_of_Polycaprolactone_via_Ring_Opening_Polymerization_Catalyzed_by_Candida_Antarctica_Lipase_B_Immobilized_onto_an_Amorphous_Silica_Support
- https://www.teachersource.com/product/thermoplastic-polymer-and-pigments/chemistry
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies