
What’s New? We have just created a handful of new chemistry activities that are based on interactive high-resolution video. These classroom-ready experiments have interactive tools so that students can perform the analysis and record data themselves. In many cases, students can even change what’s happening in the video. For example:
- In the Pressure vs Temperature activity students can analyze an ideal gas (air) to establish the idea that pressure and temperature are directly proportional and also use that proportionality to determine absolute zero. Then they can switch to a decidedly non-ideal gas (difluoroethane) to see the limitations of the ideal gas model.
- In Chemical Reactions: Magnesium and HCl students can vary the mass of the Mg.
- In the Catalase Activity Investigation students can independently change the temperature and the pH of the reaction. That freedom allows them to design and execute their own experiments.
- The Specific Heat of Water Experiment lets students measure the specific heat of water when a lightbulb is immersed into a lossy calorimeter (a glass beaker). Students can then iteratively work to improve the experimental design, e.g., wrapping the beaker in aluminum foil to limit the visible light escaping.
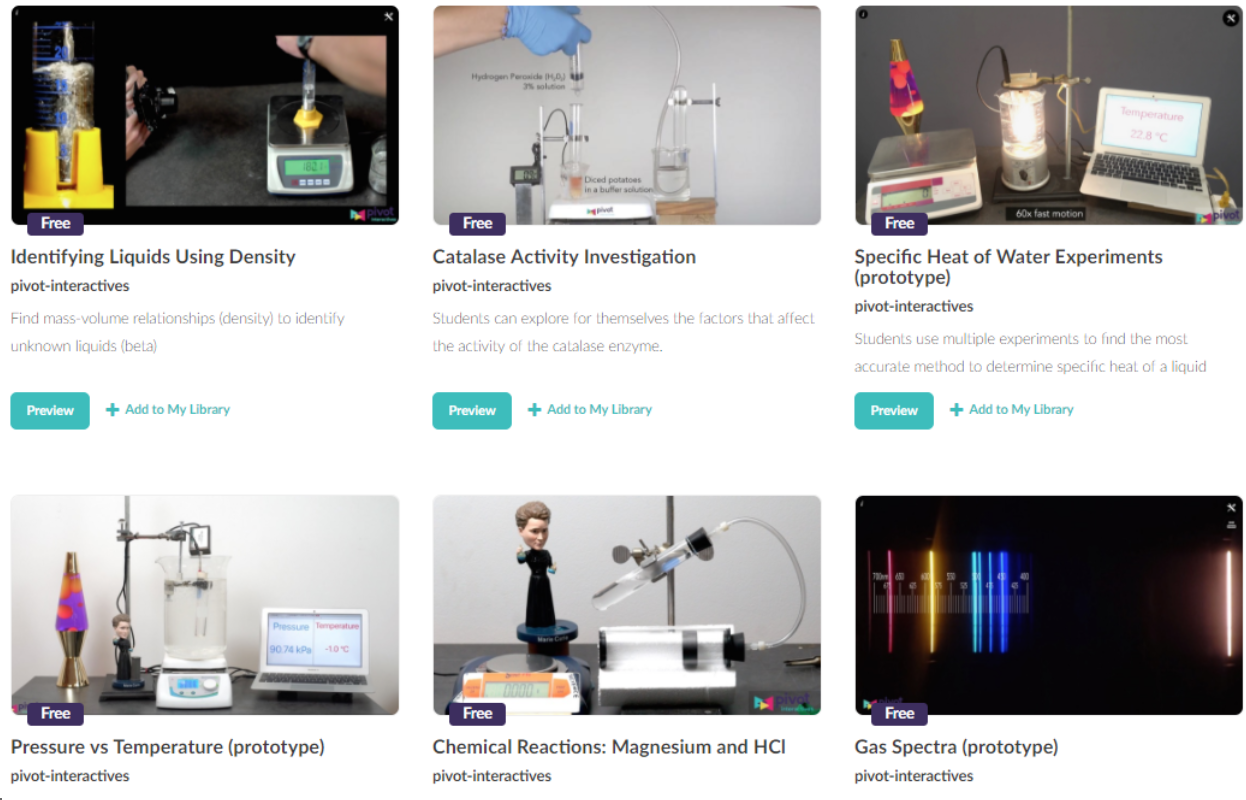
Figure 1 - Pivot interactive videos for chemistry. Freely available. (Accessed 4/15/18).
Background
We are physics teachers and several years ago we realized that it would be useful to have a set of interactive videos that would let students see the physics that was so obvious to us. At the time, no such resources existed, so we started making them ourselves and now have lots of videos that span the topics covered in Intro Physics.
Our videos are short and streamlined, showing just the phenomenon.They are not demos or tutorials; they don’t explain what’s going on. Our videos and tools allow students to do the analysis part of the experiment themselves. Students select from a palette of tools -- pH scale, stopwatch, volume graduations -- and decide what and how to measure.
Often, we record multiple versions of the event while changing one parameter at a time. That process results in a matrix of videos that students can explore on their own. Students can ask their own question and then design an experiment (by selecting the appropriate videos and performing the appropriate analysis) to answer their question.
We hope you will try out the activities!
We will be highlighting several of our chemistry videos individually in the near future on ChemEd X with the hope that this community will provide feedback. We want to know what parts you like, what could be improved and what might be missing. You might also have suggestions for how you would use them in your own curriculum.
As teachers ourselves, we’re motivated to create tools that are useful to other teachers. As physics teachers, we look to experts in chemistry education (like you) to help shape the chemistry applications of our work. We hope you will help us shape the future of Pivot Interactives.
We invite you to share your thoughts by commenting below as we hope to create a conversation within this community. You can also reach us privately at peter.bohacek@pivotinteractives.com or mattvonk@pivotinteractives.com.
Peter Bohacek, a high school teacher at Henry Sibley High School in Minnesota, and Matthew Vonk, a physics professor at the University of Wisconsin River Falls, are co-founders of Pivot interactives and co-authored this article.
NGSS
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
Modeling in 9–12 builds on K–8 and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds. Use a model to predict the relationships between systems or between components of a system.

All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 1
Suggestions for Chemistry Practicals
Dear Matthew,
Thank you for sharing your website! I find your idea of allowing students to conduct their experiments via interactive videos really unique and interesting. I'm really impressed that students can even do measurements and plot their own graphs on your website!
I'm currently working on my own video lessons at my website for students to learn chemistry online, but they are all theoretical with no "hands-on" practical experiments that students can try. It'll be wonderful if students can learn both theoretical and practical concepts online. That would truly add value to their learning.
Here are some suggestions that you might consider for your interactive lessons. They are all very common experiments that chemistry students would encounter in at high schools:
- titration reactions for acid-base reactions, eg between HCl and NaOH
- titration reaction for redox reactions, eg between KMnO4 and H2O2
- Precipitation reactions, eg test of metal cations with NaOH and NH3
- calorimetry experiments to measure enthalpy change of neutralisation for acid-base reaction
- calorimetry experiments to measure enthalpy change of combustion of organic compound, eg ethanol
Hope these suggestions might help build your existing repertoire of interactive videos.
Cheers!
Maverick Puah