
"What are we doing to help kids achieve?"
More people are getting vaccinated. The data from my region shows that schools are not superspreaders. Kids are wearing their masks. Teachers are sanitizing between classes. Some teachers can plan an entire week with hope of no major surprises. Things are inching their way back to normal. It has just been announced here that some districts are going face to face next year. Yes, there will be restrictions, masks and social distancing. The question is....is it possible to pull off labs and if so how do we do this?
I cautiously started this semester trying to figure that out. I know that not everyone reading this may be facing the same circumstances I am. I am hoping this story can be a starting point for ideas and conversations....brainstorming and problems solving. Here is one possible attempt. The context...my students were studying "Types of Reactions". The topic begged for a lab instead of me being a demonstrator or talking head. This is what I did.

Microscale, Microscale, Microscale.
We were doing a lab in which students examine a variety of reactions. They were to make observations, balance reactions and classify the types of reactions. The "wet lab" portion was primarily collecting data. Microscale made this a dream for a teacher. The set up was easy. One set up would work for several sections and could easily be sanitized between classes. (The Student Document for this lab can be found in the Supporting Information below.)
Students needed a place to do the reactions. Laminated sheets worked amazingly well. Students were able to do multiple reactions. They could easily wipe the sheet down and start over if they made a mistake and they did not have to waste the entire bell. The sheets also lended themselves well to "puddle" chemistry as I have mentioned before.*
puddle_chem.png
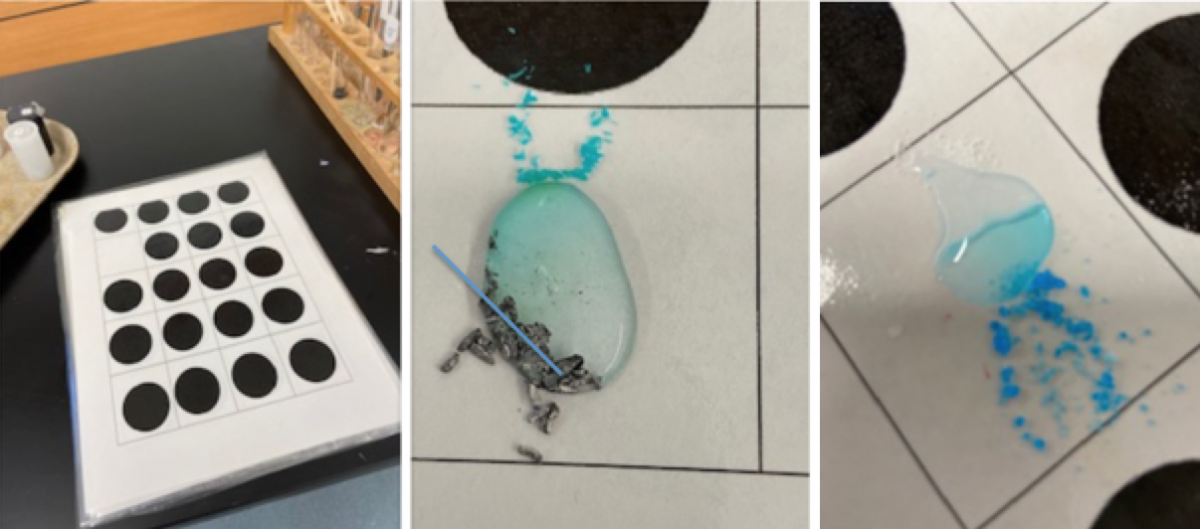
Puddle Chemistry
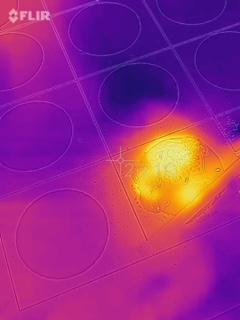
Some students noticed what they thought was heat evolving from some of the reactions. The lab did not call for it but I was able to quickly grab a FLIR camera and get a few pictures. It made for a great discussion.
We were not able to get to all of the reactions. I was able to demonstrate the last few and was able to film them with a modest set up that many classrooms possess now. This activity is one that would be easy to capture on film with little time and effort once it is set up. Overall, the students were thrilled to finally do a hands on activity. Large lab benches allowed for social distancing. The microscale portion helped with time management.
My goal for the summer is to create a spreadsheet with standards ( "I can" statements). I then plan to align all of my labs and demonstrations with the standards. I hope to have a reasonable microscale option and to safely provide some hands on experiences even if we are under restrictions. As things get better, I will be able to ease into some larger scale labs to provide students with experiences with different types of lab equipment. Interested in this plan? Stay tuned...and feel free to provide ideas or feedback.
*If you are interested in learning more about Puddle Chemistry, check out my previous blog posts.
Misconceptions and Struggles with Double Displacement reactions and dissolving...
Misconceptions and Struggles with Double Displacement reactions and dissolving...
Puddles, Dissolving and a Cool Conductivity Tester...
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.


All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 4
Microscale
Chad
Great to see you doing the microscale. You do not need to use these amounts to get good results you know. Of course, you can always add more solid if you want but it is difficult to remove it from solutions.
What is interesting is the attitude of the students. Americans are known for the BIG experiments. We have noticed that working small begins to focus the students into the work and be less concerned about what is happening around them. I don’t know whether you have other chemists, suspicious of these methods at your establishment but if the see it they often leave behind their preconceived ideas of being too small and students not able to manipulate the equipment.
I hope you notice that all these Covid test kits are microscale!
If you or any other readers any like to see more, there are a couple of videos you might like to watch.
This one is by me which is about 20 minutes. https://youtu.be/_K5WOKh6elA
The second is by Adrian Allan ( teacher from Scotland)) and myself which we did for Science on Stage https://www.science-on-stage.eu/ . Here is the film https://www.youtube.com/watch?v=LM97yXJlotQ for the Science on Stage webinar.
Thanks for the comment.
Bob, Thanks for the comment. Just so you know, I do have some people who insist that students must be introduced to certain equipment to move on or to "get ready for college". However, when I ask college professors about their programs and how to best prepare incoming students, I get a different answer from each person. My goal is to provide as much rigor and passion as possible. My reasoning is that if a student is passionate about what they are doing, they will put in the time to figure out a new method or piece of equipment when the time comes.
I still have a goal of trying to match standards with experiments this summer. If you have a repository or site for experiments, please pass them along. I have found them to be very useful. Thanks for all that you do.
Chad
By the way, loved the videos.
Microsacle
Chad
Thank you, Chad. I noticed that you write;
As things get better, I will be able to ease into some larger scale labs to provide students with experiences with different types of lab equipment. Interested in this plan?
But I hope you will retain the microscale techniques as well because they have strong Safety/ Sustainability/Green and STEM credentials plus educaional advantages. I used some thermochromic plastic to show exothermic and endothermic reactions. See https://youtu.be/-D4giFDDj5Q. Always it is the the faster moving hydrogen ions that enter the alkali. Then you can ask why. The transport is not diffusion but a different mechanism.
I also reckon I am the only person to make a precipitate of sodium chloride by mixing sat solutions of lithium chloride and sodium nitrate. See https://youtu.be/GDWJKEbIlKo . I doubt if labs would have enough lithium chloride to make 100 ml of saturated LiCl. But here you see how closely connected are precipitation reactions and solubility. Once you realise this (not mentioned in many text books and often different chapters), memory chunking goes into operation and there is a greater understanding of chemical principles.
All the time I am told that the students are focussed into the experiment and observe more. I am always grateful to a person in a workshop in the States shrieking “Gee, in a little you can see a lot”. In the UK, I have to drop the “Gee”.
We have just hosted the Royal Society of Chemistry Secondary and Further Education Group meeting followed the next day with the International Microscale Symposium and the reports you sent in this article were echoed in Japan, China, Mexico, Germany and the UK. You can see the posters on https://rscsafegroup.wordpress.com/2021-conference-posters/ .
Thank you
Bob - Thanks for the comments and the feedback. I am continually inspired and amazed by the work that you do and want to learn more about microscale. My number one goal is to try to get kids excited about chemistry and I think that much of the microscale you present will go far in achieving that goal. I do have a portion of kids that are going on to the AP curriculum here in the states. They are required to have some experience with larger scale glassware. Thanks again for you comments and your wonderful applications of microscale chemistry.