When you think back to your first experience with a chemistry experiment, what do you imagine? More likely than not, a papier-mâché volcano spewing forth the products of a mixture of baking soda and vinegar (possibly dyed with red food coloring for effect 😀) comes to mind. The chemical reaction volcano is a stalwart presence in the elementary school classroom as it is exciting, safe, and lends itself to relatively free chemistry experimentation for young children.
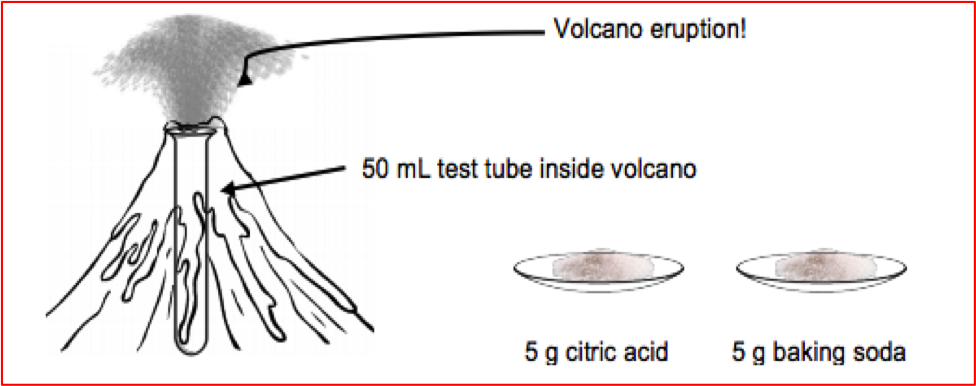
Figure 1: Image from the volcano probe student handout
Reimagining the chemical volcano
At ACCT, we wondered what could be learned about students’ thinking regarding how chemical reactions could be controlled using this familiar chemistry experiment. To accomplish this, we developed the volcano probe as a formative assessment tool for learning about how students decide which reactants to use and how to structure the conditions that their reaction occurs under (figure 1). The volcano probe formative assessment has been tested in middle school, high school, and university chemistry classes.
The volcano probe formative assessment
The volcano probe begins by showing your students a three-minute silent video, in which a student in a lab coat is considering how she will prepare a chemical demonstration that resembles a volcano for a group of second graders (video 1). The student in the lab coat ponders numerous available chemicals that are available, as well as laboratory equipment, and she chooses to use sodium bicarbonate (baking soda) and citric acid. She measures 5 g of each of these, places both in a test tube with a red triangle (“volcano” shape) behind it, and then adds 20 mL of water to the test tube. A fizzing “eruption” occurs.
Video 1: Volcano probe three-minute silent YouTube video (accessed 3/10/2020)
After students view the video, they answer questions on the Volcano Probe handout (see supporting information below), which recounts the activity in the video and lists available materials. First, students are asked “What are three different things she could do to make a bigger eruption? Explain or justify why your changes would make the eruption bigger.” Next, students are given the chemical equation for the baking soda and citric acid reaction and asked again to propose how to create a bigger “eruption”. By responding to these questions, students are revealing their thinking about chemical control, one of the six threads of the Chemical Thinking framework1.
How do students think about chemical control?
When students consider how chemical processes are controlled, they are drawing upon their intuitions about the chemical control thread of Chemical Thinking (figure 2). This thread involves thinking about how the outcomes of chemical changes can be impacted by decisions about which variables are important, and models of how chemical reactions happen. For example, we might seek to increase the output of a chemical reaction (such as in the volcano probe), to reduce the output (such as decreasing CO2 emissions), or to stabilize a process (such as keeping a furnace burning with constant heat output). Students who are novices tend to express naïve ideas about how and why things change, and what is needed to induce, halt, or feed such changes. For example, it is common for students to think that substances change because they “want to” become more stable, or that effort is always needed for things to change. They are often satisfied with these types of rationales and need to be pressed to consider and evaluate alternative explanations. Students need guidance to help them evaluate, connect, and integrate basic ideas in order to develop their chemical thinking. Multiple opportunities can be opened by teachers to reinforce such connections. There is a wide variety of questions under the umbrella of chemical control that students and teachers can discuss. For example: How can we slow down fruit decomposition? How can we maximize the usable energy output in a combustion? If we want students to develop productive ways of knowing, thinking, and acting in chemistry we need to create opportunities for them to explore the properties of the systems or processes of interest, such as the aforementioned volcano probe. In order to develop an understanding of the complexities of this question, students need to consider why and how things change, and what kinds of variables can be controlled. Students need the opportunity to predict impacts of different changes they could make and try them out to learn how to think about chemical control effectively.
Figure 2: Six threads of chemical thinking
As teachers, we can leverage fruitful discussions about chemical control with students to elicit more about students' initial ideas and ways of reasoning. From asking students to clarify their own thinking, we can identify students’ own productive ideas that we can capitalize on to advance their thinking. We can also help students to recognize limitations that intuitive ways of reasoning can impose on their development of our targeted understandings. Eliciting students’ own ideas puts us in a better position to build upon students’ productive resources, Thus, to foster growth of chemical control thinking, we can first characterize how students intuitively think about how to control the properties and behaviors of matter, and what types of implicit assumptions students make about what causes a system to change.2
An example from some students’ written work from the volcano probe reveals the types of ideas that students may volunteer related to this particular formative assessment. For example, in response to the question “What further ideas do you have on things that the student could change to make the eruption bigger? Explain or justify... “ one student said “Given the information, she should use less baking soda so she could create more aqueous solution (which we want) and less gas (which can’t even be seen given the point of the volcano experiment).” In this case, the student is expressing the idea that by reducing the baking soda, less gas would be produced. The student may (or may not) know that the decomposition of sodium bicarbonate produces CO2 gas, and perhaps might be thinking that by reducing the amount of baking soda, more solution would bubble up, so more volcano lava would bubble up. The student has focused on the role that sodium bicarbonate plays in the gas production, so that reducing it could help to control the reaction. This is a productive resource of the student’s that can be built upon. It could be interesting to elicit further information from the student, perhaps by asking how the student thinks the eruption happens. It could also be worthwhile to advance the student toward the science story by encouraging them to try their hypothesis that using less baking soda reduces the amount of gas produced by the reaction. It is important to recognize that there are multiple productive pathways that teachers may take in order to further their students’ chemical thinking. We at ACCT don’t prescribe one path over another, rather we seek to help teachers make more purposeful in-the-moment decisions with students in our classrooms.
This is Part 1 of a multipart series from the Assessing for Change in Chemical Thinking (ACCT) project. We, the members of ACCT (Becca, Greg, Hannah, Michael, Rob, Scott), represent a NSF-funded collaboration (NSF awards DRL-1222624 and DRL-1221494) between university researchers, graduate and postdoctoral students, and high school and middle school teachers. ACCT focuses on fostering chemical thinking in middle school, high school and undergraduate classrooms through strategic formative assessment usage. To accomplish this we develop resources, tools, and professional development for teachers of chemistry to foster students’ chemical thinking. We also study how chemistry teachers’ reasoning about formative assessment changes and how chemistry teachers shift to emphasize formative assessment as a lever for change. By working with teachers nationwide, we believe that we can help teachers reimagine the way that they think about chemistry, and develop more purposeful, productive and engaging ways of interacting with their students to help them learn. (If you would like to learn more about us, check out our prior ChemEd X blog post, ChemEd X conference page and a JChemEd article about how we collaborate). Our work focuses on Chemical Thinking and Formative Assessment as two major frameworks for professional development and research.
ACCT would love to connect with you!
As a group, we at ACCT are looking to connect with teachers nationwide to build an educator community around formative assessment and chemical thinking, as well as to share the resources that we have built and are continuing to develop. You can explore more about the ACCT project on our ACCT landing page, and we welcome you to reach out to us at ACCTProject@umb.edu.
References
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23. (accessed 3/10/2020)
- Talanquer V., (2019), Why and how to foster chemical thinking? Unpublished manuscript.
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about natural and designed worlds. Arguments may also come from current scientific or historical episodes in science.
Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.