
Pharmaceutical magnesium ion is ingested as a low-dosage dietary supplement or as a high-dosage osmotic laxative. Water-soluble magnesium citrate is a safe and effective pharmaceutical compound for both purposes. As a laxative, magnesium citrate is supplied as a water-soluble dry powder that does not contain any magnesium citrate until it has been dissolved in water. The magnesium citrate is produced by a chemical reaction in the aqueous solution.
This article describes the chemistry of two magnesium citrate laxative products: Picolax®1 and Citramag®2. Magnesium ion is an essential nutrient for humans3, found in many foods. It is safely absorbed from the digestive tract. Citrate ion is fully absorbed from the digestive tract and is also safe to ingest. Magnesium citrate is a safe and effective nutritional supplement. How then can it also be a laxative? Magnesium ion absorbance from the digestive tract is limited to an amount in the same order of magnitude as the daily nutritional requirement. The laxative dosage is nearly ten times as much as the nutritional requirement. The unabsorbed magnesium ions of the laxative dose and their counter anions remain undigested and reach the colon, where they exert an osmotic laxative effect4. The magnesium ion in the colon is otherwise not harmful. A purge laxative consists of two laxative doses, each including a stimulant of bowel action, the second taken after a few-hour interval.
Adult Magnesium and Magnesium Citrate Dosages

The magnesium citrate laxative products that are the subjects of this article are notable in that each is supplied as a dry-powder mixture that does not contain magnesium citrate. Each is a reagent mixture that reacts chemically when dissolved in water, forming the pharmaceutical as an aqueous solution for ingestion. Solid magnesium citrate is hygroscopic, absorbing water from the air. For use as a pharmaceutical substance, solid magnesium citrate requires the addition of a processing aid. Using solid magnesium citrate, manufacturers can produce low-dose tablets, caplets, or capsules, which are swallowed, but not laxatives. High-dose laxatives need a large mass and, therefore, a large volume of dry, free-flowing powder to satisfy production requirements. This powder must also be fully soluble in water to satisfy patient acceptance. The processing aid is not soluble in water. Solid magnesium citrate containing a processing aid does not satisfy either requirement. Pharmaceutical manufacturers have found a way to get around this problem by making a mixture of an alkaline magnesium compound with anhydrous citric acid. This mixture reacts when added to water to produce a solution of magnesium citrate for ingestion.
Two Magnesium Citrate Laxatives - Active Ingredient Content Only

light magnesium oxide = MgO
heavy magnesium oxide = Mg5(CO3)4(OH)2⋅5 H2O
Laxatives are potentially dangerous pharmaceutical products. The manufacturers are required by regulation to disclose the names and quantities of the active ingredients to the users. From the supplied information, it is possible to write balanced equations for the reactions and to calculate the theoretical yields of magnesium citrate formed, the magnesium dosages, and the limiting and excess reagent quantities. The two case studies presented here can be used in teaching, for assignments, or as review exercises. A resource PDF file titled Questions and Information for Students is available for download in the supplemental materials. The chemistries of Picolax® and Citramag® are summarized below.
Magnesium Citrate Laxatives Formulated as Magnesium Oxide / Anhydrous Citric Acid Mixtures
Ferring Pharmaceuticals of Switzerland manufactures this formulation and sells it around the world under at least four trademarked names: as Picolax® (in Europe), as Pico-Salax® (in Canada), as Picosalax® (in Australia), and as Prepopik® (in the U.S.) An ingredient list available for Picolax® has the most complete description of these products6. Sodium picosulfate is a stimulant of bowel activity7. For a very readable and complete description of the action of this pharmaceutical, see Dr. Robert J. Hillsden’s 2011 editorial in the Canadian Journal of Gastroenterology8.
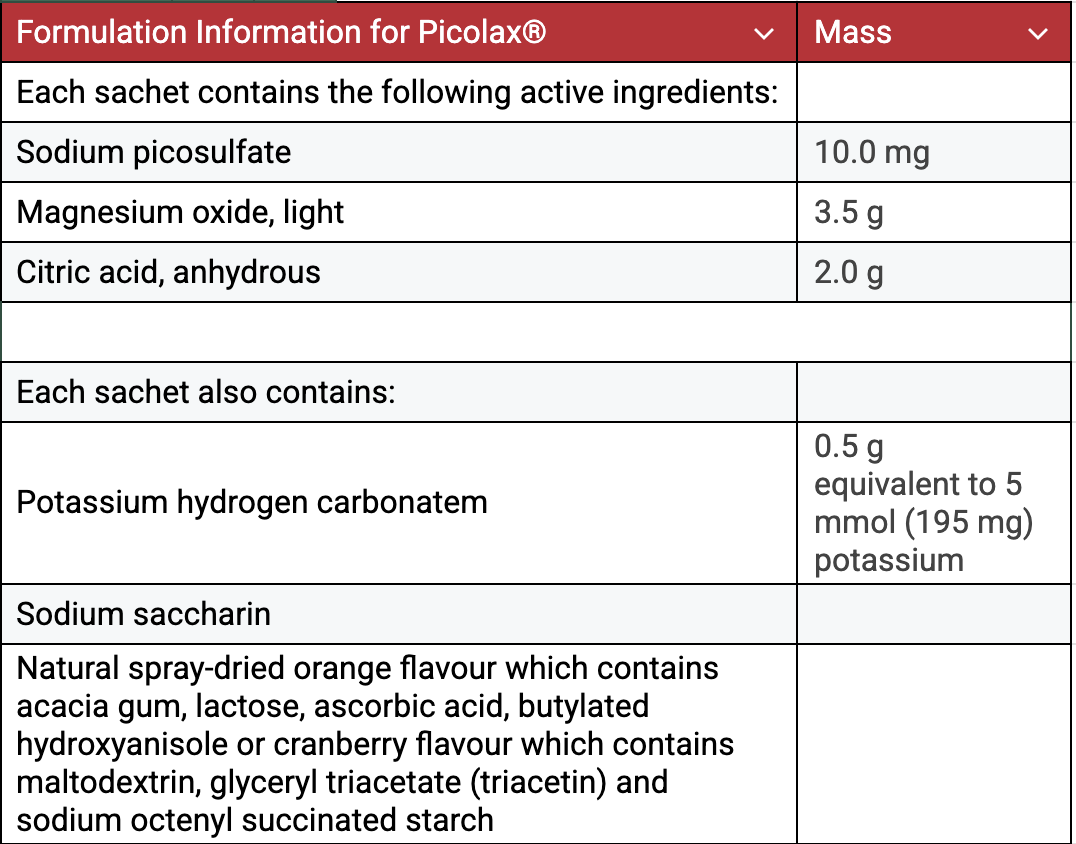
When the dosage of this powder is added to water, four chemical reactions occur. You can view a video of this process occurring on YouTube9. Both the absolute amount of each substance present and the relative amounts are important in ensuring the safety and efficacy of the pharmaceutical.
In this summary of the chemistry occurring, it will be assumed that only complete formulas and complete equations will be used at an introductory level. At a later stage in a course, ionic equations could also be written for these reactions. In this article, for writing equations and calculating yields, magnesium citrate will be taken to have the formula Mg3(C6H5O7)2. It will be called magnesium citrate rather than trimagnesium citrate or magnesium citrate 3:2 as used in Wikipedia10.
Chemical Reactions
1. Solid magnesium oxide (magnesia) reacts immediately with water to form insoluble magnesium hydroxide (milk of magnesia). This is observed as a milky precipitate:
MgO(s) + H2O → Mg(OH)2(s) (This is Equation 1)
2. Soluble citric acid dissolves in the water, reacting with the solid magnesium hydroxide, which has formed to form soluble magnesium citrate:
3 Mg(OH)2(s) + 2 H3C6H5O7 (aq) → Mg3(C6H5O7)2(aq) + 6 H2O (This is Equation 2)
3. Solid magnesium oxide also reacts with the dissolved citric acid, forming soluble magnesium citrate:
3 MgO(s) + 2 H3C6H5O7(aq) → Mg3(C6H5O7)2 (aq) + 3 H2O (This is Equation 3).
Equation 3 is the overall equation used for quantity calculations.
Note that Equation 3 is the sum of Equations 1 and 2.
Equation 3 = (3 × Equation 1) + Equation 2
4. The dissolved citric acid also reacts with the potassium hydrogen carbonate (potassium bicarbonate), which is very soluble in water. This reaction produces effervescence due to the production of carbon dioxide and dissolved potassium ion. This potassium ion is useful as it can replace the potassium ion lost in the fluid excreted from the colon:
3 KHCO3(aq) + H3C6H5O7(aq) → K3C6H5O7(aq) + 3 CO2(g) + 3 H2O
To understand the chemical and physiological rationales behind the formulation of the magnesium citrate component of these pharmaceutical mixtures, it will be necessary to determine whether magnesium oxide or citric acid is the limiting reagent and how much of the excess reagent remains unreacted. One calculation will be shown in complete detail, after which the results of each calculation will be stated. The given amounts, 3.5 g of magnesium oxide and 12 g of citric acid, will be taken as exact values, and extra digits will be carried through the calculations.
In the real world of pharmaceutical manufacture, the amount of each component in each dosage of a heterogeneous powder is variable. The rules for how much variation is allowed in North American pharmaceuticals are set by the FDA / USP. The rules are somewhat complex11. To oversimplify, the actual content of each active ingredient in a pill or powder can be expected to be within ±10 % of the stated value 95 % of the time. Reducing the variability is costly, but batches that do not comply must be discarded.
Values Required for Calculations
|
Citric Acid (a triprotic acid) 192.1 g/mol C6H8O7 or H3C6H5O7 |
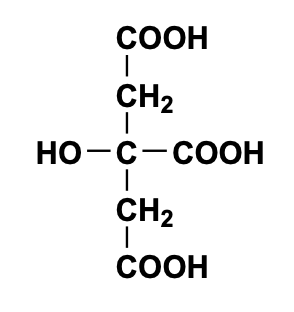 |
pKa Values 3.14 4.75 6.40 |
Magnesium Oxide MgO = 40.31 g/mol (60.31 % Mg by mass)
Magnesium Citrate Mg3(C6H5O7)2 = 451.1 g/mol
Potassium Bicarbonate KHCO3 = 100.1 g/mol
Quantity Calculations
1. Theoretical yield of magnesium citrate from 3.5 g of magnesium oxide:
3.5 g MgO = 3.5 g MgO × (1 mol MgO / 40.31 g MgO) = 0.08683 mol MgO
= 0.08683 mol MgO × (1 mol Mg3(C6H5O7)2 / 3 mol MgO) = 0.02894 mol Mg3(C6H5O7)2
= 0.02894 mol Mg3(C6H5O7)2 × (451.1 g Mg3(C6H5O7)2 / 1 mol Mg3(C6H5O7)2 )
= 13.06 g Mg3(C6H5O7)2
2. Theoretical yield of magnesium citrate from 12 g of anhydrous citric acid:
= 14.09 g Mg3(C6H5O7)2
Therefore the magnesium oxide is the limiting reagent.
3. Amount of citric acid required to react with 3.5 g of magnesium oxide:
= 11.12 g citric acid
4. Amount of citric acid required to react with 0.50 g KHCO3 : (The amount 0.50 g is given in the formulation information.)
= 0.32 g citric acid
5. Excess amount of citric acid:
= 12 g – 11.12 g – 0.32 g = 0.56 g citric acid
6. Total dosage of magnesium per sachet:
= 3.5 g MgO × (60.31/100) = 2.1 g Mg
Magnesium Citrate Laxative Formulated as a Heavy Magnesium Carbonate / Anhydrous Citric Acid Mixture

Cambridge Healthcare Products produces Citramag®purgative. This pharmaceutical product is utilized in the UK (United Kingdom) by the NHS (National Health Service)12.
Each sachet of Citramag® contains 11.57 g of heavy magnesium carbonate and 17.79 g of anhydrous citric acid.
The abbreviation BP on the package stands for British Pharmacopeia, the UK equivalent of USP.
Chemical Reaction
A single chemical reaction occurs when the dosage of powder is dissolved in water solution. One dosage is used for a laxative effect; two doses taken at a few-hour interval, each with a bowel stimulant taken at the same time, is used for a purgative effect. Both the absolute amount of each substance present and the relative amounts are important in ensuring the safety and efficacy of the pharmaceutical.
Values Required for Calculation
|
Citric Acid (a triprotic acid) 192.1 g/mol C6H8O7 or H3C6H5O7 |
 |
pKa Values 3.14 4.75 6.40 |
Heavy Magnesium Carbonate Mg5(CO3)4(OH)2⋅5 H2O = 485.6 g/mol (25.03 % Mg by mass)
As the very water-soluble citric acid dissolves, it reacts with the solid heavy magnesium carbonate to form soluble magnesium citrate, carbon dioxide, and water:
3 Mg5(CO3)4(OH)2⋅5 H2O(s) + 10 H3C6H5O7 (aq) → 5 Mg3(C6H5O7)2(aq) + 12 CO2(g) + 33 H2O
Quantity Calculations
To understand the chemical and physiological rationales behind the formulation of the magnesium citrate component of the laxative/purgative, it will be necessary to determine whether heavy magnesium carbonate or anhydrous citric acid is the limiting reagent, and how much of the excess reagent remains unreacted. The given amounts 11.57 g of heavy magnesium carbonate and 17.79 g of anhydrous citric acid, will be taken as exact values, and extra digits will be carried through the calculations.
Reactant and product masses of the above equation:

1. Mass of anhydrous citric acid required to react with 11.57 g of heavy magnesium carbonate:
11.57 g Mg5(CO3)4(OH)2⋅5 H2O
= 11.57 g Mg5(CO3)4(OH)2⋅5 H2O × (1921 g H3C6H5O7 / 1456.8 g Mg5(CO3)4(OH)2⋅5 H2O)
= 15.26 g H3C6H5O7
Therefore the heavy magnesium carbonate is the limiting reagent.
2. Excess amount of citric acid remaining unreacted:
= 17.79 g – 15.26 g = 2.53 g citric acid
Excess Citric Acid, Potassium Ion, and Sodium Saccharin Amounts in the Ingested Pharmaceuticals
Having calculated the amounts of the excess citric acid remaining when one sachet of each type of laxative is utilized, it is possible to calculate the concentrations of the citric acid in the solution ingested. These concentration values may then be compared to those typical in fruit juices and synthetic soft drinks (13). However, in the pharmaceutical solutions, there is also a large amount of citrate ion present. These solutions are therefore buffered at a much higher pH value than any juice or drink. They are safe to ingest.
Excess Citric Acid Concentration in the Ingested Magnesium Oxide Laxative
This product contains nominally about 0.56 g of excess citric acid dissolved in each 150 mL of aqueous solution ingested.
Concentration of Citric Acid = (0.56 g / .150 L) = 3.7 g / L
Excess Citric Acid Concentration in the Ingested Heavy Magnesium Carbonate Laxative
This product contains nominally about 2.53 g of excess citric acid dissolved in each 200 mL of aqueous solution ingested.
Concentration of Citric Acid = (2.53 g / .200 L) = 12.7 g / L
Potassium Ion Concentration in the Ingested Magnesium Oxide Laxative
The magnesium oxide laxative lists 0.5 g potassium hydrogen carbonate (potassium bicarbonate) as an inactive ingredient. The potassium hydrogen carbonate reacts with citric acid, providing 5 mmol or 195 mg of dissolved potassium ions as an electrolyte replacement, and carbon dioxide gas to carbonate the solution, making it more palatable. A loss of potassium ions is a major concern in a purge or in many intestinal illnesses.
Concentration of potassium ions in the ingested solution:
= 5 mmol / 0.150 L = 33.3 mmol / L
The WHO (World Health Organization) formulation for oral rehydration14suggests a concentration of 20 mmol/L for potassium when rehydrating, with an acceptable range of 15 – 25 mmol/L. A concentration of 33 mmol/L is slightly above the recommended value. By comparison to the 195 mg of potassium per dose, a 360 mL bottle of Gatorade contains 50 mg of potassium15.
Sodium Saccharin Quantity in the Ingested Magnesium Oxide Laxative
It is possible to estimate the amount of sodium saccharin in a dose of laxative.
Assume that the medication should taste as sweet as apple juice16 or orange juice.
The typical glucose content of a fruit juice is about 10 % m/v.
Therefore, a 150 mL dose of laxative should taste as if it contains 15 g of glucose.
Convert 15 g glucose to sucrose = 11 g of sucrose17.
Sodium saccharin is approximately 500 times as sweet (mass/mass) as sucrose.
Taking the estimate of 11 g = 11,000 mg of sucrose per dose of the medication, and a sweetness ratio of 1 to 500 for sucrose to saccharin, the estimated amount of saccharin is 22 mg = 0.022 g per sachet.
Magnesium Ion in Human Nutrition
Magnesium ion is an essential nutrient for human nutrition. For authoritative information about magnesium in foods and magnesium supplementation, see the web page “Magnesium – Fact Sheet for Health Professionals”, National Institute for Health, Office of Dietary Supplements3
Magnesium ions may be used as a pharmaceutical by ingestion of either solid magnesium oxide (magnesia) or an aqueous suspension of magnesium hydroxide (milk of magnesia). However, using these compounds, the ingestion of an alkaline salt imposes a nutritional imbalance. For routine daily use of small supplemental doses or for one-time ingestion of a large laxative or purgative dose, ingestion of a nearly neutral aqueous solution is preferred. One preferred medication is an aqueous solution of magnesium citrate. The citrate ion is easily absorbed and is not hazardous.
At low dosages of magnesium ion, these solutions function as absorbable supplements.
Low dosage here means 50 to 400 mg of magnesium ion per day, depending on age, body mass, and nutritional category. This quantity of magnesium ion, taken as magnesium citrate, is usually fully absorbable from the digestive tract.
Osmotic Laxative Effect of Magnesium Ion in the Bowel
Absorbance of magnesium from the intestinal tract is limited to roughly the amount required for good health. The remainder of the magnesium ions and the counter ions remain in the intestinal tract and reach the bowel. Although magnesium ion is non-toxic and has no ill effects actively, the addition of these ions increases the number of dissolved particles in the bowel. The walls of the bowel are permeable to water. Osmosis causes an increase in the net flow of water into the bowel. The magnitude of this effect depends on the amount of magnesium ingested. When a laxative or purgative dosage of magnesium is ingested, the result is very large. Combined with a stimulant to bowel activity, the contents of the bowel will thereby be completely emptied.
Adult Magnesium Dosages

A mild laxative effect utilizing magnesium may be achieved at least cost by ingesting an aqueous suspension of magnesium hydroxide (milk of magnesia). This medication, however, is very alkaline and is unsafe at the larger doses that are required in a purgative.
Using Magnesium Citrate in Low-Dose Magnesium Supplement Products Webber Naturals Magnesium Citrate - Bottle Label Information:
Each capsule contains magnesium (as citrate) 150 mg.
Nonmedicinal ingredients: microcrystalline cellulose, vegetable-grade magnesium stearate (lubricant).
Magnesium citrate, called magnesium citrate (3:2) by Wikipedia, is an ionic salt. It is relatively soluble in water and slightly hygroscopic. It is difficult to produce commercially with a precise magnesium content18, and difficult to use in the manufacturing of pharmaceuticals without mixing with processing additives. It is difficult to keep dry in storage. Magnesium citrate can be used by the manufacturers of fixed low-dosage magnesium supplements by the addition of processing aids such as micro-crystalline cellulose (MCC)19. This tactic allows the manufacturer to produce tablets or capsules that are swallowed whole, reducing the storage issues.
The additive MCC is insoluble in water and hydrophobic. Using such a mixture for a high dosage laxative product would not be commercially acceptable, since the powder mixture cannot be kept dry in storage, would lump and would not flow well from its package into a glass of water for dissolution and ingestion, and would not fully dissolve. The consumer would not readily accept such a product.
A dry powder is the preferred choice for almost all pharmaceuticals. The powder may be formed into tablets, placed in capsules, or placed in sachets to be dissolved or suspended in water. The advantages are many: less packaging; less costly transport; longer shelf life; ease of unit dosing, to name a few. Relative to supplying the medication in a bottle as a suspension or solution, there is far less danger of dosage error, spilling, or spoilage.
Light Magnesium Oxide - MgO
The term light here refers to the low temperature of production of the magnesium oxide.
Wikipedia: “Magnesium oxide is produced by the calcination of magnesium carbonate or magnesium hydroxide. ……. Calcining at different temperatures produces magnesium oxide of different reactivity. High temperatures 1500 – 2000 °C diminish the available surface area and produces dead-burned (often called dead-burnt) magnesia, an unreactive form used as a refractory. Calcining temperatures 1000 – 1500 °C produce hard-burned magnesia, which has limited reactivity and calcining at lower temperature (700–1000 °C) produces light-burned magnesia, a reactive form, also known as caustic calcined magnesia.”20
Light magnesium oxide has numerous uses and is a major industrial chemical, $US 1.59 billion in 202321, 22. Pharmaceuticals is the fastest-growing use sector. Light magnesium oxide can be milled to a fine powder that is stable when dried at a temperature above 100 °C. It does not absorb moisture from the air. It can then be blended with dried anhydrous citric acid to form a stable mixture which does not react until added to liquid water. This mixture is suitable for pharmaceutical use.
Heavy Magnesium Carbonate - Mg5(CO3)4(OH)2⋅5 H2O
Magnesium carbonate is an important industrial chemical23. The market size was about $US 0.33 billion in 2023. But magnesium carbonate or MgCO3 as such is not easy to manufacture. Rather, there is a family of products referred to nominally as magnesium carbonate, where each member of the family has a sectorial name. Lohman Minerals, a producer of magnesium salts, lists eight magnesium carbonate mineral formulas24. Only one of these minerals, magnesite (MgCO3), has neither water of hydration nor hydroxide ion content. The other seven all have either one or both as an integral part of the crystalline structure.
The difficulty of manufacturing anhydrous MgCO3 by precipitation from an aqueous solution results from two major factors. Firstly, the very small 2+ magnesium ion has a strong mutual affinity to the oxygen of the water molecule. The magnesium ion is highly solvated in aqueous solution, and most of its salts are precipitated as hydrates (e.g., MgSO4.7H2O). Secondly, concentrated carbonate ion solutions are very basic because carbonate ion is a weak base. It reacts with water, producing hydroxide ions, which can co-precipitate with carbonate ions.
H2O + CO32-(aq) ⇌ OH-(aq) + HCO3– (aq)
One member of the magnesium carbonate family of compounds that may be produced in a pure form with reproducible composition is the hydrated double salt Mg5(CO3)4(OH)2⋅5 H2O25. This salt hydrate is very insoluble in water and has a specific crystalline structure. In industry, it is named heavy magnesium carbonate. It occurs in geological formations as the mineral dypingite. There are numerous locations around the world where this mineral occurs, including in the US and in Canada26.
Heavy magnesium carbonate is a double salt27:
Mg5(CO3)4(OH)2⋅5 H2O = (MgCO3)4⋅Mg(OH)2⋅5 H2O (double salt)
A double salt is a complex ionic solid with a crystalline lattice whose composition corresponds to a mixture of two simpler salts. Writing the formula as a double salt can be helpful in determining quantities for a synthesis reaction, or for writing balanced equations, but it does not provide any guide to the physical structure of the substance. The three-dimensional structure is best determined by X-ray crystallography.
Heavy magnesium carbonate is available as a food-grade and a pharmaceutical-grade substance. It is probably the substance present in magnesium carbonate and magnesium citrate type supplements. It is certainly used in the formulation of Citramag®.
Heavy magnesium carbonate can be milled to a fine powder that is stable to drying at a temperature above 100 ° C. It does not absorb moisture from the air. It can then be blended with dried anhydrous citric acid to form a stable mixture which does not react until dissolved in water. This mixture is suitable for pharmaceutical use.
Equation for the Synthesis of Heavy Magnesium Carbonate
Heavy magnesium carbonate is synthesized by reacting a soluble magnesium salt, such as the chloride or sulfate, with sodium carbonate ina very hot water solution. The product is very insoluble. The reaction equation is the sum of five reactions occurring in the solution.
5 MgSO4(aq) + 5 Na2CO3(aq) + 6 H2O → (MgCO3)4⋅Mg(OH)2⋅5 H2O + 5 Na2SO4(aq) + CO2(g)
See Section 9 of the problem set for the derivation of this equation.
Sources

