
Implementing a Process-Oriented Guided Inquiry Learning (POGIL) style recrystallization activity provides students with an opportunity to actively engage in the learning process while reinforcing key chemical concepts such as polarity, solubility, and purification techniques. Research has shown that a POGIL approach promotes improved conceptual understanding, critical thinking, and teamwork skills compared to traditional laboratory approaches1,2. Incorporating preparatory modules further scaffolds student learning, as pre-laboratory activities have been demonstrated to enhance student confidence and performance in laboratory settings3,4. The focus on student collaboration through defined roles also aligns with evidence that structured group work improves both accountability and communication skills in STEM learning environments5. Finally, connecting the hands-on procedure with reflection questions encourages metacognitive development, which has been identified as a critical factor in fostering deep learning in chemistry laboratories6.
This activity engages students in a POGIL approach to recrystallization, designed to integrate concepts of polarity, solubility, and the fundamental principles underlying purification of solids. Building upon a prior week’s traditional, hands-on recrystallization experiment, students are now challenged to apply their knowledge in a more collaborative and inquiry-driven format.
Before beginning the activity, students completed three preparatory modules that emphasized the relationship between polarity and solubility, the effect of temperature on solubility, and the use of mixed melting points to identify recrystallized solids. In class, students are organized into groups of three, with designated roles of manager, recorder, and presenter to promote accountability and structured teamwork. Each group receives an unknown solid and is tasked with designing and executing a procedure for its recrystallization and subsequent identification.
The activity concludes with guided reflection questions, allowing students to evaluate both their experimental decisions and their understanding of the underlying chemical principles. In doing so, this activity provides a scaffolded transition from traditional laboratory instruction toward active, student-centered learning, emphasizing critical thinking, collaboration, and the application of theoretical concepts in practice.
This experiment is safe, economical, and allows instructors to adjust based on the availability of solvents and solids used as unknowns. The methodology used will serve to better prepare students for upper-level chemistry laboratories and undergraduate research experiences. The reagents listed (solids for recrystallization and solvents) were used by the author in an undergraduate organic chemistry 1 laboratory. Instructors may change both solid samples and solvents.
Time required: 2 laboratory sessions, each of 2 ½ - 3 hours. In the first session, students followed a prescribed method for recrystallization of a known compound (acetanilide). This first session may be substituted by a review video or other resource that introduces the concepts of recrystallization. The activity may be broken down into shorter sessions by separating the recrystallizations and melting point determination activities.
|
150 mL beakers |
Büchner funnel |
|
Erlenmeyer flask |
Funnel |
|
Test tubes |
Mel-Temp apparatus |
|
Test tube rack |
Hot plate (with magnetic stirring) |
|
Thermometer |
Stirring bar |
|
Vacuum filter flask |
Laboratory balance |
|
Reagents:
|
Solvents: |
|
Benzoic acid |
Water |
|
Acetanilide |
Ethanol |
|
Vanillin |
Hexane |
|
Lauric acid |
|
|
Salicylic acid |
|
|
Ascorbic acid |
Table 1: Solid samples used in the activity, along with the theoretical melting point and ideal solvent for recrystallization.
|
Unknown number |
Ideal solvent |
Theoretical Melting Point7-12 (°C) |
Mixed Melting Point (°C) |
Percent recovery % |
Identity |
|
1 |
Water |
122 |
|
|
Benzoic acid |
|
2 |
Water |
114 |
|
|
Acetanilide |
|
3 |
Water |
82 |
|
|
Vanillin |
|
4 |
Ethanol |
43 |
|
|
Lauric acid |
|
5 |
Water |
159 |
|
|
Salicylic acid |
|
6 |
Ethanol |
191 |
|
|
Ascorbic acid |
In this activity, students will work collaboratively in groups of three, with each member assuming a designated role (manager, recorder, presenter) to guide their inquiry. The experiment begins with guided models and questions that help students understand the relationships between polarity, solubility, and solvent selection. First, students will analyze the provided data and rank solvents according to polarity. Using the principle of “like dissolves like,” each group will predict which solvents are most suitable for dissolving an organic solid. Next, they will evaluate temperature-dependent solubility data to determine which solvent provides the optimal profile for recrystallization, which is low solubility at room temperature but high solubility at elevated temperatures. After this, students will meet with the instructor to discuss their answers and reasoning.
Once solvent selection is complete, each group will be assigned an unknown solid. Students will develop their own procedure for the recrystallization of their unknown sample. Although each group will arrive at slightly different procedures, in broad terms, they should all start with testing a number of available solvents to determine the ideal solvent to be used in their experiment. After this step, each group will proceed to delineate and execute a procedure for recrystallizing their solid compound. Each group will attempt to dissolve their sample in the chosen solvent, heat the mixture to obtain a saturated hot solution, and then carefully cool it to allow recrystallization. If necessary, they may use hot filtration to remove insoluble impurities. After crystals have formed, the product will be collected by vacuum filtration, dried, and weighed to determine the percent recovery.
To assess purity and confirm the compound’s identity, students will measure the melting point of both the crude and recrystallized solids. A sharp, narrow melting point range indicates a high-purity sample. Additionally, students will carry out a mixed melting point test with a suspected reference compound. If the mixture displays the same sharp range, the identity is confirmed; if the range broadens, the unknown and reference compounds are different.
Throughout the process, students will document their procedure, observations, and reasoning for each decision they make. At the conclusion of the experiment, each group will share their findings with the class, including the unknown number, solvent used, yield, melting point data, and proposed identity of the compound. Final reports will incorporate both individual group data and the pooled results from all groups, encouraging students to compare approaches and reflect on factors affecting purity and yield.
References:
- Moog, R. S.; Spencer, J. N. Process Oriented Guided Inquiry Learning. In Process Oriented Guided Inquiry Learning (POGIL); Moog, R. S., Spencer, J. N., Eds.; American Chemical Society: Washington, DC, 2008; pp 1–13. https://doi.org/10.1021/bk-2008-0994.ch001
- Minderhout, V.; Loertscher, J. Lecture-Free Biochemistry: A Process Oriented Guided Inquiry Approach. Biochem. Mol. Biol. Educ. 2007, 35 (3), 172–180. https://doi.org/10.1002/bmb.39
- Miller, T.; Birch, M.; Mauthner, M.; Jessop, J., Eds. Ethics in Qualitative Research, 2nd ed.; SAGE Publications: London, 2012.
- Sandi-Urena, S.; Cooper, M.; Stevens, R. R. Effect of Cooperative and Problem-Based Lab Instruction on Metacognition and Problem-Solving Skills. J. Chem. Educ. 2012, 89 (6), 700–706. https://doi.org/10.1021/ed1011844
- Eberlein, T.; Kampmeier, J.; Minderhout, V.; Moog, R. S.; Platt, T.; Varma-Nelson, P.; White, H. B.Pedagogies of Engagement in Science: A Comparison of PBL, POGIL, and PLTL. Biochem. Mol. Biol. Educ.2008, 36 (4), 262–273. https://doi.org/10.1002/bmb.20204
- Cooper, M. M.; Stowe, R. L. Chemistry Education Research — From Personal Empiricism to Evidence, Theory, and Informed Practice. Chem. Rev. 2018, 118 (12), 6053–6087. https://doi.org/10.1021/acs.chemrev.8b00020
- https://pubchem.ncbi.nlm.nih.gov/compound/Benzoic-Acid (accessed 10/02/2025).
- https://pubchem.ncbi.nlm.nih.gov/compound/Acetanilide (accessed 10/02/2025).
- https://pubchem.ncbi.nlm.nih.gov/compound/Vanillin (accessed 10/02/2025).
- https://pubchem.ncbi.nlm.nih.gov/compound/Lauric-Acid (accessed 10/02/2025).
- https://pubchem.ncbi.nlm.nih.gov/compound/Salicylic-Acid (accessed 10/02/2025).
- https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbic-acid (accessed 10/02/2025).
In this guided-inquiry activity, each student group is responsible for developing the procedure for recrystallization and identification of an unknown solid. As they work through and answer the model questions and interpret the data provided, they will decide how to purify the sample and how to confirm its identity using melting point and mixed melting point determination. The numbered steps below represent the standard procedure that each team should ultimately arrive at through their reasoning and discussion.
- Record team information
- Write down the names of all group members and their assigned roles (manager, recorder, presenter). * The responsibilities of each role are described in the student handout.
- Review solvent concept
- Work through the guided questions on polarity, solubility, and identification of a recrystallized solid.
- Determine the ideal solvent for the recrystallization of their unknown sample
- Using a small test tube for every solvent to be tested, place a small amount of the solid sample in the test tube, and enough solvent to cover the solid sample.
- Record the solubility (low, high) of the solid sample in each solvent.
- If the solubility is high, discard the solvent.
- If the solubility is low, place it in a hot water bath for a few minutes. Record the solubility of the solid sample in each of the remaining solvents.
- Choose the most appropriate solvent for recrystallization based on temperature-dependent solubility trends.
- Dissolve the unknown sample
- Place the unknown solid in an Erlenmeyer flask.
- Add a minimum amount of the chosen solvent.
- Heat the mixture gently until the solid dissolves completely.
- Remove insoluble impurities (if needed)
- While hot, filter the solution quickly through fluted filter paper into a clean flask.
- Keep the solution hot during this process to prevent premature crystallization.
- Crystallize the compound
- Allow the hot solution to cool slowly to room temperature without disturbing it.
- If necessary, place the flask in an ice bath to encourage further crystallization.
- Isolate and dry the crystals
- Collect the solid crystals by vacuum filtration.
- Rinse the crystals with a small amount of cold solvent to wash away impurities.
- Leave the crystals to dry completely.
- Determine yield
- Record the mass of the dry crystals.
- Calculate the percent recovery based on the original sample mass.
- Measure melting points
- Determine the melting point range of the crude (impure) solid.
- Measure the melting point of the recrystallized solid and compare it to literature values. Choose the (tentative) identity of the solid based on potential identities provided by the instructor.
- Perform a mixed melting point test
- Mix a small amount of the recrystallized unknown with an equal amount of a known reference compound.
- Grind the mixture thoroughly to ensure uniform blending.
- Measure the melting point range of the mixture.
- Interpret results:
- A sharp range close to the reference value suggests the two are the same compound.
- A broadened or depressed range indicates they are different.
- Identify the compound
- Compare the melting point data (pure and mixed) to known values.
- Propose the most likely identity of the unknown.
- Share and compile results
- Report the group’s data (unknown number, solvent used, percent yield, melting point data, and identity).
- Gather results from all groups to include in the final report.
- Reflect on the process
- Complete the post-lab reflection questions about solvent choice, purity, yield, and sources of error.
Model 1: relationship between polarity and solubility
When choosing an appropriate solvent to dissolve a solid organic compound, organic chemists follow the rule of "likes dissolves likes". What this means is that polar compounds will dissolve with polar solvents, and non-polar compounds will dissolve with non-polar solvents.
Questions:
1. Which solvent will you choose to dissolve benzoic acid? 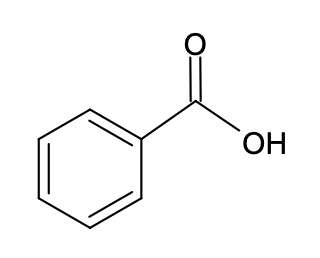 Explain.
Explain.
a. water H2O
b. ethanol 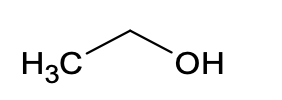
c. hexane 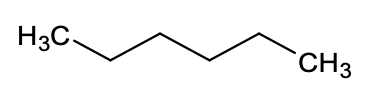
2. Rank the following solvents in order of increasing polarity (least polar to most polar)
a. water
b. diethyl ether 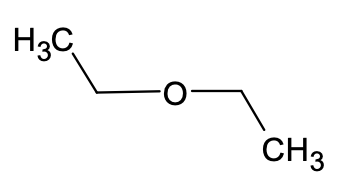
c. ethanol 
d. dichloromethane 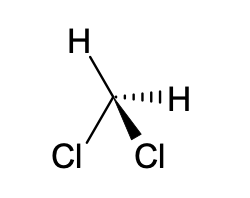
e. hexane 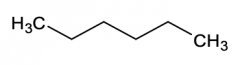
Model 2: choosing the best solvent to recrystallization a solid: relationship with temperature.
With regards to temperature, the best solvent for a given compound will show no solubility at low temperatures, and high solubility at high temperatures. Although there are other considerations, the temperature/solubility dependence is one of the most important that will determine the suitability of a given solvent to recrystallize a given solid organic compound.
Question:
1. The following table shows the solubility data for benzoic acid under various solvents. Based on this data, which solvent would you choose for recrystallization. Explain.
|
Solubility of Benzoic acid as a function of temperature (g/100 mL) |
|||
|
Temperature (°C) |
Water |
Ethanol |
Benzene |
|
20 |
0.29 |
6.8 |
12.0 |
|
40 |
0.56 |
12.0 |
17.5 |
|
60 |
1.1 |
19.0 |
23.0 |
|
80 |
2.3 |
28.0 |
30.0 |
|
100 |
6.8 |
36.0 |
36.5 |
Model 3: characterization using melting point and mixed melting point.
The melting point for a given compound is measured as a range at which the compound undergoes melting. A narrow melting point (ΔT≈ 2) is a good indication of high-purity crystals. An alternative method used to identify a solid is called the mixed melting point. In it, the unknown sample is mixed with a known compound in equal amounts. If they are the same compound, the melting point range remains narrow. If they are different compounds, one would act as an impurity on the other, leading to a dramatic increase in their melting temperature range.
Question:
- A student recrystallized an unknown solid and attempted to both determine its purity and identity. The student believed that the solid was Salicylic acid, with a theoretical melting point of 156 °C. They performed a melting point determination for the unknown solid, the recrystallized solid, and for a 1:1 mixture of the unknown solid with Salicylic acid. The following data shows the melting range for (a) impure crystals, (b) recrystallized, and (c) using the mixed melting point for the unknown solid. Would you consider the crystals to be pure? Was the solid, in fact Salicylic acid? Explain.
|
Mass of unknown sample (g) |
2.57 |
|
Theoretical melting point of Salicylic acid (°C) |
156 |
|
Melting point of recrystallized solid (°C) |
151 – 153 |
|
Melting point of recrystallized solid (°C) *using mixed-melting-point method* |
144 - 156 |
Activity: what is the identity of my unknown sample?
In this activity, each group will purify and characterize a given compound, using the principles of recrystallization. After completing the task, each group will report their data to the class, including the unknown number, chosen solvent, yield, and melting point range. Before turning in the report, each group must have all the data from every group included in their respective reports.
Write down the procedure your team follows and include it in your report. Explain your reasoning behind the chosen procedure.
Reflection (post lab) questions
1. How did you choose the best solvent for the recrystallization? Describe in detail.
2. Would you expect a different outcome if your team had chosen a different solvent? Explain.
3. What factors would affect the purity of the recrystallized sample?
4. What factors would affect the yield of your sample?
Instructor notes:
If possible, students should carry out this experiment in small groups of 2 – 4, with each group member having a unique role in the group (manager, recorder, presenter).
Allow enough time for students to work out the modules before proceeding with the experiment of recrystallizing their unknown compound.
If needed, this laboratory can be split into two or three sessions, with convenient stopping points after the theoretical exercises (Modules 1-3), and after recrystallization of the solid sample. Allowing time for the samples to dry after recrystallization will allow for more accurate melting point ranges.
Table 1 shows the solid samples used by the author in an undergraduate organic chemistry 1 laboratory course. Substitutions for solid samples and solvents based on criteria determined by the instructor should not affect the overall laboratory experience.
While obtaining a high yield is important, the main objective of this experiment is the guided-inquiry approach, for students to gain hands-on experience in the design of an experimental procedure. Instructors may adapt the activity and grade accordingly.
Ethanol and Hexane are volatile organic solvents, caution should be exercised, and all work should be performed in a well-ventilated area.
Written by Dr. Daniel Rivera
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
