Co-authors: Rachel Barnard*, Samantha Cass, and Ryan Sweeder (*: corresponding author)
Lyman Briggs College, Michigan State University
When it comes to learning some of the more quantitative aspects of chemistry, practice can be very helpful. For this reason, students often seek to prepare themselves by seeking out additional practice problems. By repeating similar problems, the students hope to develop the skills that allow them to then be successful on new questions that they will see on their exam. To many novices in a field, small changes to questions can make a question feel completely different and require the student to approach the problem anew (for one example, see An and Carr, 2017). In this way, a titration problem that involves sodium hydroxide reacting with acetic acid may seem completely different from ammonia reacting with sulfuric acid. Experts in the field though will readily recognize both as stoichiometric acid-base reactions. In fact, it is this deeper level of abstract understanding that experts possess that allow instructors to readily develop new quiz or exam questions.
In this article, we describe efforts to help novice students build a deeper abstract understanding which is a universal goal of instructors. The classroom activity involves students using a method to develop and then solve novel quantitative chemistry problems following a MadLibsTM style (see image below). The method incorporates student choice and metacognitive reflection while allowing for relatively quick feedback. We used this activity as a cumulative activity for students in General Chemistry 2, college-level lecture course; yet it would be readily adaptable to other instructional situations. The complete classroom activity is available in the Supporting Information.
Context and Motivation:
We had previously incorporated an end-of-the-semester assignment designed around students generating their own exam-worthy chemistry questions. This assignment was intentionally open-ended so as to provide maximum flexibility to students to come up with unique questions. We found that it did the opposite; these earlier iterations lacked scaffolding which meant that students struggled to create viable, unique questions. Instead most student-generated questions were very simple, or did not make any chemical sense. However, after a year of co-writing new student worksheets thanks to the COVID-19 pandemic, we created a new student-generated questions activity that provided more structure. The additional structure was added so students did not feel overwhelmed with the possibilities while simultaneously still creating an activity that met our learning objectives. It should be noted that although a large emphasis in the course is on developing chemistry core ideas while using science practices, this new review activity does not emphasize either. Instead it focuses on giving students the opportunity to create novel questions that also engage students to reflect on their own learning and understanding.
Since many of the calculation questions on old worksheets followed patterns that were common to that question's content, we felt like we were completing a MadLibTM. For example, if we wanted to create a new question that assessed students' ability to calculate the pH of a weak acid, we would choose a weak acid, look up its pKa, and choose a starting concentration and the rest of the question was quite similar no matter what was chosen for each variable. However, this MadLibsTM analogy held an insight to how to help students understand the underlying patterns to similar problems. The analogy of MadLibsTM (where the players pick specific words within a category) provided an opportunity to suitably scaffold the process of question writing to help highlight the underlying similarities between questions.
It is these similarities in quantitative chemistry questions that are often not apparent to students, but are the mark of expert level understanding. We have in the past used a method we call "Steps In Problem Solving" (SIPS) in our General Chemistry 1 and 2 lecture courses to help students approach unfamiliar quantitative problems. This method is intended to help students see and appreciate these patterns and to reduce their cognitive load while solving such questions (Dunlosky, et al, 2013). Solving the problems conceptually also allows the students to more readily explore how different questions may have similar underlying concepts. A video explanation of the SIPS method can be viewed below.
Relevant Precedent:
The idea of student-generated test questions is by no means a new classroom assessment technique. Angelo and Cross (1993), for example, describe using it to assess students' course-related knowledge and skills in applying knowledge. Yet it is a technique that is underused in higher education that can have positive affective impacts such as decreasing test anxiety (Aflalo, 2021). Although the creation of student-generated instructional materials has been used as a class project (Zurcher, et al, 2016; Coppola & Pontrello, 2020), we were seeking a simpler version that did not require rounds of instructor feedback. For these reasons, the MadLibsTM approach to scaffolding seemed appropriate as the inherent structure of the game provided a base structure to provide support.
Since students already had assigned working groups, the fact that there were options gave students the chance to have discussions about how different selections might alter the approach to solving the resultant problem. In fact, we sought to force these conversations by adding follow up questions designed to engage students in the metacognitive reflection to see how small changes in questions might impact the approach to problem solving. In the preparation of this essay, we even discovered that the MadLibsTM website suggests students create their own MadLibs as a cumulative activity that meets a variety of learning goals (MadLibs in the Classroom). Since the MadLibsTM website is outside of our usual instructional materials reference sources, we figured it would still be worth sharing this approach.
Implementation:
We adopted the MadLibsTM style worksheet as a General Chemistry 2 recitation activity designed to review for the cumulative final quiz (though the idea could be used in many settings). We wanted the worksheet to address the major topic areas from the semester. Students were asked to:
(1) construct exam questions via a series of scaffolded statements and question(s),
(2) construct the SIPS map for the question(s), and
(3) answer a set of "thinking critically" questions where they were asked to reflect on their problem solving, including how changing variables would have changed their approach.
The assignment was available to students prior to the weekly recitation meeting. They worked on it outside of class time with their assigned group of 3-6 peers prior to the weekly recitation meeting. Undergraduate Learning Assistants (ULA) are embedded in the teaching team and support students during the active lectures and recitation meetings each week. During the recitation meeting, the ULAs would review each group's work and answer any questions. Students received a score for completing the assignment.
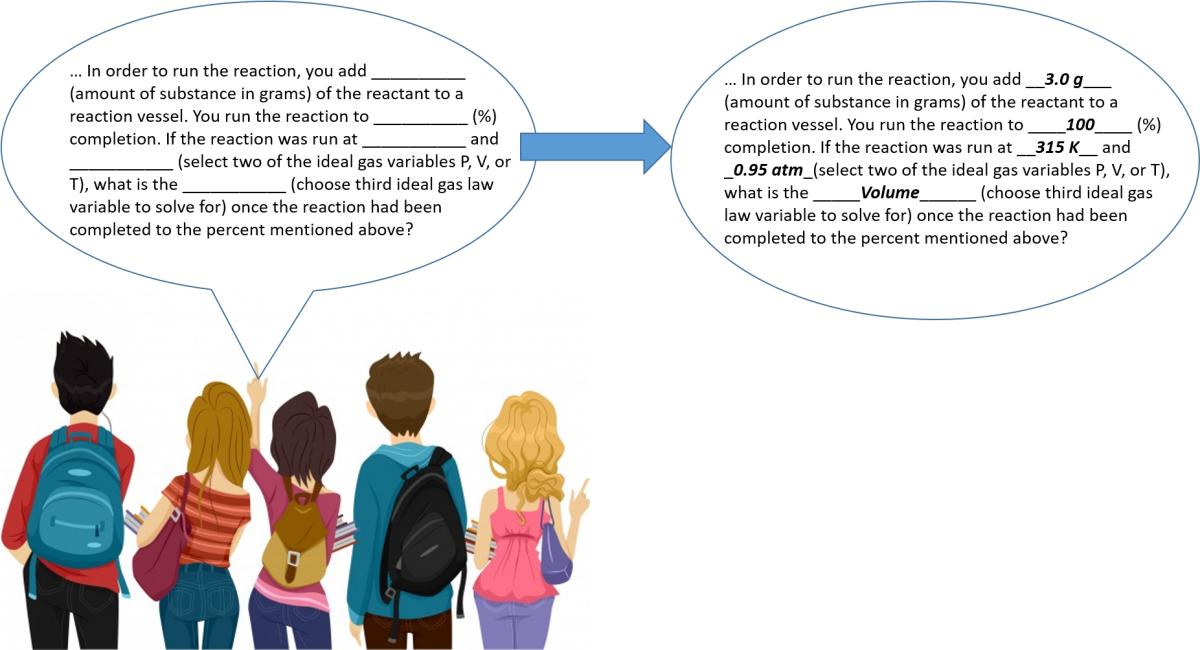
For the assignment, student groups were instructed to choose two content areas to focus on from the following list: thermodynamics, gas laws, colligative properties, integrated rate laws, and equilibrium. These are most of the units taught in our General Chemistry 2 course. Within each chosen topics they then choose a sub-focus (a reaction, a colligative property, or a compound). Then there was a series of sentences to complete with blanks. For example, a gas law question is shown below.
After creating a question, student groups were asked to draw out a SIPS map for how they would solve for a particular variable. By asking for the process and not the final value, we could:
(1) review the skills of making SIPS maps,
(2) allow the students to focus on the general steps to solve a quantitative problem, and
(3) make it easier for our ULAs to give feedback on the steps while not having to prepare for every possible question the students might generate.
Finally, students were asked a series of "thinking critically" questions. These were intended to help students see that changing parts of the question (for exmaple, reversing the reaction, or changing a variable) would influence the problem-solving process, but that core aspects in solving the problem would remain the same. To reflect on their learning, the final question asked on each topic area is: "What part(s) of this exercise did you find the most challenging? Be sure to go over those in your ULA conference."
Let's take a look at two student group responses. The first (1) involves equilibrium and the reactant chosen, ammonium bromide. The second involves thermodynamics and the chosen gas phase reaction, 2SO3(g) → 2SO2(g) + O2(g). Bold text below indicates student group responses.
(1) Equilibrium: Write out a chemical equation for how it reacts with water: NH4Br + H2O ⇆ NH4+ + Br-. The bromide anion is a spectator anion and does not affect pH.
It would create a(n) acidic (acidic/basic/neutral) solution. NH4+ + H2O ⇆ NH3 + H3O+; Here, since NH4+ has a positive charge, it "wants to give away" a proton to become neutral and is acting as "HA" from our typical acid-base equation. It will give away a proton to H2O, which is acting as a base, to form the neutral conjugate base NH3 plus hydronium, which makes our solution acidic.
Now suppose your group is asked to create a buffer involving the compound selected with pH of 6 (select a pH). [Note, you may need to look up either a Ka or Kb relevant for your compound here.] Your group adds 10 mol NH4Br (amount of compound A-D) to create 20L solution (volume of solution with units). The substance your group decides to add to make the buffer is NH3(aq) (identity of substance).
Draw a SIPS map for how you would determine how much this substance (in grams) you would need to add in order to achieve the desired pH:
Thinking critically: Suppose your group had to choose a different approach to create the buffer above. Identify a different substance that you could add to create a buffer. How does the problem change? How does it stay the same?
You could add a strong acid or base (e.g. HCl) to create a buffer. You'd end up using the limiting reagent method instead. If you were adding HCl to your already-existing buffer, you'd have to figure out how much of the weak base is reacting with HCl, and how much conjugate acid that'd create as a result.
(2) Thermodynamics: 2SO3(g) → 2SO2(g) + O2(g)
Upon inspection of the reaction chosen you determine that the reaction would have a positive (positive/negative) entropy. Explain your reasoning here: Entropy is a measure of disorder within a system. Because we are going from one molecule to two molecules, we are increasing the amount of collisions occurring within the system. Changes in entropy can result from an increase in moles, increase in temperature, or a change in state of matter.
Your group decides to calculate the enthalpy of the reaction by using standard enthalpies of formation (select a way to calculate the enthalpy). What information do you need to perform the calculation? The enthalpies of formation for SO3, SO2, and O2. ΔHoreaction = ΣΔHfoproducts - ΣΔHforeactants
Draw a SIPS map for how you would solve this problem:
Based on the sign of the entropy and the enthalpy, the reaction you selected is non-spontaneous (spontaneous or non-spontaneous) at low temps. ΔG = ΔH - TΔS; ΔG = (+) - (+)(+)
Thinking critically: How would your answer above (for spontaneous/non-spontaneous) change if the reaction was flipped? How do you know? What part(s) of this exercise did you find the most challenging? Be sure to go over those in your LA conference.
If this reaction was flipped, it would become spontaneous at low temperatures. ΔHf would become -197.78 kJ/mol, and entropy would become negative because disorder is increasing. I know because of Gibbs free energy equation, ΔG = (-) - (+)(-).
Take-Aways for Implementation:
In reflection and via discussion with our ULAs, we found several important items for those considering using this in their classroom. First, having students work through the activity in their assigned groups in preparation for their recitation meeting gave students sufficient time to do any necessary reviewing that was needed. Second, providing time for students to work on a question prior to recitation was very helpful for both ensuring that the students had enough time to thoughtfully develop their problem. And this allowed them to focus on their reflection questions with the ULAs.
Third, because students are generating novel questions, it is impossible to know every answer to support our ULAs in providing feedback for students. Using the SIPS maps instead of calculating answers allowed us to short circuit that problem by focusing on the underlying conceptual approach. This allowed us to support the ULAs and required students to solve the problem in a more conceptual manner which puts an emphasis on the underlying methodology rather than simply finding a numerical answer.
Finally, we noted that many of the groups could try to simplify their questions by making strategic selections of variables to make the questions easier (such as selecting 0 or 100% yields). By asking students to reflect on other possible ways to vary the questions in the final portion of the set of questions for each topic forces students to consider all of the variables and how they work together in a more comprehensive sense. This also ensured that students considered different versions of the question (for example starting with products and determining the amount of reactants) and thought through how this would relate to the SIPS map.
Rachel Barnard, Samantha Cass, and Ryan Sweeder
Lyman Briggs College, Michigan State University
CITATIONS:
Aflalo. E. (2021) Students generating questions as a way of learning. Active Learning in Higher Education 22(1): 63-75. https://doi.org/10.1177/1469787418769120
An, D., & Carr, M. (2017) Learning styles theory fails to explain learning and achievement: Recommendations for alternative approaches. Personality and Individual Differences 116: 410–416. https://doi.org/10.1016/j.paid.2017.04.050
Angelo, T. A., & Cross, K. P. (1993) Classroom assessment techniques: A handbook for college teachers. Jossey-Bass Publishers.
Coppola, B. P., & Pontrello, J. K. (2020) Student-Generated Instructional Materials. In J. J. Mintzes & E. M. Walter (Eds.), Active Learning in College Science: The Case for Evidence-Based Practice (pp. 385–407). Springer International Publishing. https://doi.org/10.1007/978-3-030-33600-4_24
Dunlosky, J., Rawson, K. A., Marsh, E. J., Nathan, M. J., & Willingham, D. T. (2013) Improving Students' Learning With Effective Learning Techniques. Psychological Science in the Public Interest. 14(1): 4-58. https://doi.org/10.1177/1529100612453266
Madlibs in the Classroom (n.d.) https://www.madlibs.com/MadLibs-Teachers-Guide-nocrops.pdf
Zurcher, D. M., Phadke, S., Coppola, B. P., & McNeil, A. J. (2016) Using Student-Generated Instructional Materials in an e-Homework Platform. Journal of Chemical Education, 93(11): 1871–1878. https://doi.org/10.1021/acs.jchemed.6b00384
ACKNOWLEDGEMENTS:
The authors would like to thank the Undergraduate Learning Assistants who both provided feedback on the assignment and helped implement it in the recitation.