
Kermit the Frog sings, “It’s not easy being green” but the role of Green Chemistry is to show people not only is it easier, but also the way we need to be doing chemistry. Green Chemistry is defined as, “The design of chemical products and processes that reduce and/or eliminate the use or generation of hazardous substances. This approach requires an open and interdisciplinary view of material and product design, applying the principle that it is better to consider waste and hazard prevention options during the design and development phase, rather than disposing, treating and handling waste and hazardous chemicals after a process or material has been developed." 1
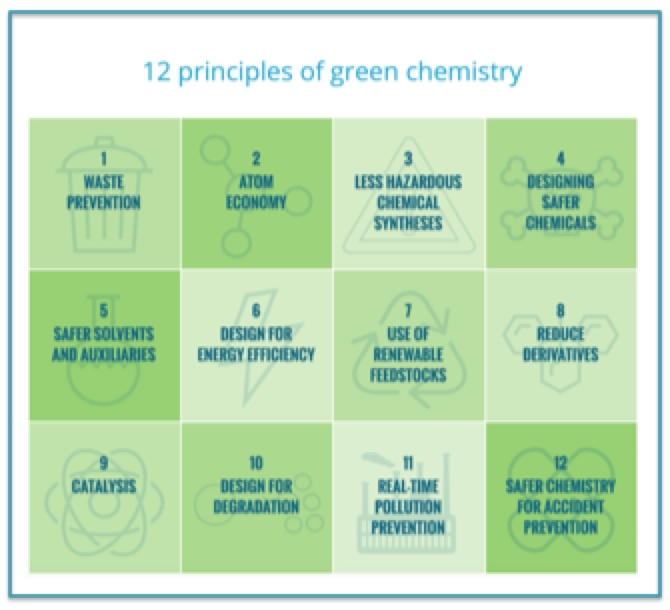
Figure 1: "Twelve Principles of Green Chemistry" - Image from Beyond Benign website 1
Dr. John Warner and Dr. Paul Anastas published a book called “Green Chemistry: Theory and Practice” in 2000. One of the most noteworthy concepts from the book is the outline of the 12 Principles of Green Chemistry (see image 1). I was first introduced to the idea in 2013 when I took an online graduate credit class about the topic. From there, I have done a lot of work with spreading the word of Green Chemistry mainly through working with a non-profit company out of Massachusetts called Beyond Benign. Their mission is to develop and disseminate resources that empower educators, students and the community to practice sustainability through green chemistry.
Reasons Why I Use Green Chemistry
Below are the top 5 reasons why I use Green Chemistry in my high school chemistry classes.
Reason #1 - Student Safety
It is a top priority for me to provide safe instruction for my students. Using a potentially hazardous chemical or method is not worth the risk, especially if there is a safer alternative.
Reason #2 - Environmental Protection
It is important to consider the environment and do whatever we can to be good stewards to our planet. Green chemistry is one way to open the communication about protecting our environment.
Reason #3 - 21st Century Skills/NGSS Aligned
Green chemistry naturally lends itself to problem solving, critical thinking, argumentation, and collaboration. These skills are important for our students no matter what field they pursue. They also line up well with NGSS expectations.
Reason #4 - Real-world Connections
As chemistry teachers, I think we somehow feel pressure to use 'real' chemicals in our labs. In reality, there are so many more connections our students can make when we are able to use household substances. This also helps students realize that those household substances are 'real' chemicals too!
Reason #5 - Career Opportunities
Environmental science, sustainability and greener practices are being adopted by most industry leaders. Exposing students to the types of thinking needed to problem solve how we can make our labs more green will be beneficial and open up potential career interests.2,3
Replacement Labs
One of the easiest ways to start to bring Green Chemistry into your classroom is to check out the many replacement labs offered on the Beyond Benign website. Below is a highlight of a few of my favorites. I hope you will check out the full list of replacement labs.

Image 2: Box of Colorflame candles
Flame Tests-Atomic Emission Spectra4: For all of the replacement labs, it is important for students to understand what traditional lab is being replaced and why. In regards to flame tests, the After the Rainbow video put out by the U.S. Chemical Safety Board is a powerful example of the need for teachers to understand the principles of Green Chemistry. The replacement lab uses the Colorflame birthday candles or Colorflame tea lights to teach the same principle (see image 2). It is easy, relatable, cost effective and most of all safe.
Endothermic/Exothermic5: Another popular replacement lab is to demonstrate endothermic and exothermic chemical reactions using Pixie Stix candy, water, beef liver (or potato) and hydrogen peroxide. This is a replacement for chemicals such as calcium chloride and ammonium nitrate which are often used to demonstrate these principles. The safety warning for ammonium nitrate states, “Danger! Strong oxidizer. Contact with other material may cause a fire. Causes eye, skin, and respiratory tract irritation. May cause methemoglobinemia. Hygroscopic (absorbs moisture from the air). Ammonium nitrate when contaminated with oil, charcoal, or other organic materials should be considered an explosive capable of detonation by combustion or by explosion of adjacent explosive materials.” 6
Le Chatelier’s Principle7: The replacement lab for demonstrating Le Chatelier’s Principle uses household substances like tea, vinegar, ammonia, starch and iodine to show the shift in chemical equilibrium. This procedure is much safer than using cobalt chloride or potassium chromate. Watch the Beyond Benign video of this lab being performed below.
Video 1: Green Chemistry: Le Chatelier's Principle & Dynamic Equilibrium8
Einstein stated, “Problems cannot be solved with the same level of awareness that created them.” It’s time we stop being reactive to our approach to chemical safety and start being proactive by using the green chemistry principles to ensure a safe and sustainable learning environment for our students.
Green Chemistry Resources
1. About Green Chemistry, Beyond Benign website (accessed 8/5/19)
2. Jobs Associated With Green Chemistry, Chron website (accessed 8/5/19)
3. Yfke Haber, Green Means Go For Careers in Chemistry, Feb 2013 Chemistry World website (accessed 8/5/19)
4. Download the Flame Tests-Atomic Emission Spectra activity, Beyond Benign website, 2017 (accessed 8/5/19)
5. Download the Endothermic/Exothermic activity, Beyond Benign website, 2017 (accessed 8/5/19)
6. Ammonium nitrate Material Safety Data Sheet, Fisher Scientific Education website (accessed 8/5/19)
7. Download the Le Chatelier’s Principle activity, Beyond Benign website, 2017 (accessed 8/5/19)
8. Green Chemistry: Le Chatelier's Principle & Dynamic Equilibrium Video, Fisher Scientific Education YouTube Channel, 2011 (accessed 8/5/19)
*The American Chemical Society provides Green Chemistry resources on their website. There are several documents available for free download that can be used with students.(accessed 8/5/19)
**Preview Image is modified from a freely available image found on Pexels.com https://www.pexels.com/photo/clear-light-bulb-planter-on-gray-rock-1108572/(accessed 8/5/19)
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
Assessment is limited to chemical reactions involving main group elements and combustion reactions.
Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.
Students who demonstrate understanding can apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.
Assessment is limited to simple reactions in which there are only two reactants; evidence from temperature, concentration, and rate data; and qualitative relationships between rate and temperature.
Emphasis is on student reasoning that focuses on the number and energy of collisions between molecules.
Students who demonstrate understanding can refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can refine the design of a chemical system by specifying a change in conditions that would produce increased amounts of products at equilibrium.
Assessment is limited to specifying the change in only one variable at a time. Assessment does not include calculating equilibrium constants and concentrations.
Emphasis is on the application of Le Chatelier’s Principle and on refining designs of chemical reaction systems, including descriptions of the connection between changes made at the macroscopic level and what happens at the molecular level. Examples of designs could include different ways to increase product formation including adding reactants or removing products.
