
Crystalline solids are composed of atoms, ions, or molecules that are arranged in an extended regular repeating array. Huge numbers of units of the repeating array are required to build each cubic millimeter of most simple solids.
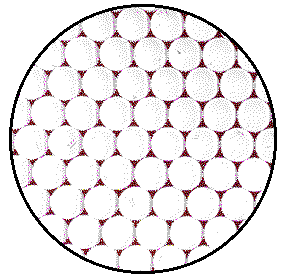
The figure shows a model of a plane of atoms in a crystal of platinum metal. To model the actual number of atoms in the 2 mm circle on the crystal, the magnified image would need to be about 8,000,000 atoms wide rather than 8 atoms wide as drawn. A model covered with a layer of 1/2 inch marbles would require a circle with a diameter of about 60 miles.

Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software