|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
| Silver Mirror - Tollens Test for Aldehydes |
 |

View Slide Thumbnails |
|

View Next Movie |
|
Voiceover
Aqueous solutions of glucose, silver nitrate plus ammonium nitrate, and sodium hydroxide are mixed in a Florence flask. The flask is stoppered and swirled to evenly coat the flask with the mixture. A silver mirror soon coats the flask as the Ag(I) ion is reduced to Ag metal and glucose is oxidized to gluconic acid.
Discussion
A silver mirror coats the flask because the Ag(I) ion is reduced to Ag(0) as Ag(I) oxidizes glucose to gluconic acid.
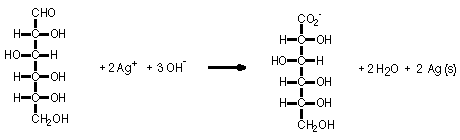
Silver Mirror - Tollens Test for Aldehydes |
|
|
|
|
Safety note: Tollens’ reagent may explode on storage. Demonstrators repeating this should use only freshly prepared reagent and dispose of the reactions’ products immediately after the demonstration.
|
Citation:
|
|
| |
Kemp, M.
|
J. Chem. Educ. 1981, 58, pp 655-656.
|
|
Design, Text and Demonstrator:
|
|
| |
Gary Trammell
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Videographer/Editor:
|
|
| |
Steve Dykema
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Voice:
|
|
| |
Margaret Biddle
|
University of Wisconsin - Madison, Madison, WI 53706
|
|
Audio Production:
|
|
| |
Greg Minix
|
University of Wisconsin - Madison, College of Engineering, Madison, WI 53706
|
| |
Jerrold J. Jacobsen
|
University of Wisconsin - Madison, Madison, WI 53706
|
|

