| Silver Mirror - Tollens Test for Aldehydes |
| |
Click a thumbnail picture below to view a larger version. |

View Movie |
|
Narrative
Aqueous solutions of glucose, silver nitrate plus ammonium nitrate, and sodium hydroxide are mixed in a Florence flask. The flask is stoppered and swirled to evenly coat the flask with the mixture. A silver mirror soon coats the flask as the Ag(I) ion is reduced to Ag metal and glucose is oxidized to gluconic acid.
Discussion
A silver mirror coats the flask because the Ag(I) ion is reduced to Ag(0) as Ag(I) oxidizes glucose to gluconic acid.
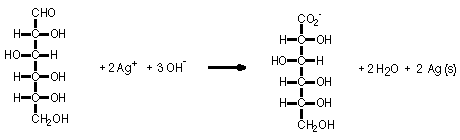
Silver Mirror - Tollens Test for Aldehydes |
|
|
|
|
Safety note: Tollens’ reagent may explode on storage. Demonstrators repeating this should use only freshly prepared reagent and dispose of the reactions’ products immediately after the demonstration.
|

