 Suppose we want to manufacture a ton of aluminumin at a space colony on the moon. Two "recipes" would be: Suppose we want to manufacture a ton of aluminumin at a space colony on the moon. Two "recipes" would be:
Take ten tons of anorthosite (CaSi2Al2O8, a common lunar mineral). Melt in a solar furnace at 2000 kelvin in vacuum. After all silicon has been removed by evaporation as silicon dioxide, quickly cool the melt to give a glassy solid. Remove the glassy material, grind, and mix with sulfuric acid. Use a centrifuge to separate the aluminum-bearing liquid which results. Mix with sodium sulfate and heat to 500 kelvin. Use another centrifuge to separate the resulting sodium aluminum sulfate. Bake it at 1100 kelvin to produce a mixture of alumina and sodium sulfate; wash out the latter with water. Mix the alumina with carbon, and react the mixture with chlorine. This gives aluminum chloride. Put the aluminum chloride through electrolysis. Result: one ton of molten aluminum.
Alternatively, the calcium aluminate produced in the melting step in the previous recipe can be readily separated into aluminum, calcium and oxygen by electrolysis after dissolving the calcium aluminate in a CaF2-LiF molten salt electrolyte.
I. Write chemical equations for each process involved in the manufacture of aluminum.
II. Identify the virtue and problems associated with both of these lunar manufacturing techniques.
1. Write a balanced net ionic equation for the reaction that occurs when the weak acid HF (hydrofluoric acid) reacts with an aqueous solution of magnesium nitrate. Include the physical state (s, l, g, aq) of each reactant and product species.
2. Assuming that the magnesium nitrate solution is mixed with excess hydrofluoric acid, draw a submicroscopic diagram of the situation once all the magnesium nitrate has reacted. Use the following to represent the molecules and ions involved.
 water molecule water molecule |
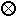 hydrogen ion hydrogen ion |
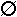 fluoride ion fluoride ion |
 HF molecule HF molecule |
 magnesium ion magnesium ion |
 nitrate ion nitrate ion |
|



