
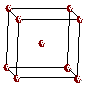
A body-centered cubic metal crystallizes with the metal atoms located at the lattice points of a body-centered cubic space lattice.
Click the links on the right to alternate between the lattice and the atom views; and to view two alternate unit cells. View definitions of underlined words in the text by clicking them.
The unit cell commonly selected for a body-centered cubic metal is a cube with a lattice point at each corner and a lattice point in the center of the cube. Although it may not be obvious in the drawing, all lattice points within a body-centered structure are surrounded by a cube of other lattice points.

Body-Centered Cubic Space Lattice


Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software