Continuous and Discrete Light Sources | |
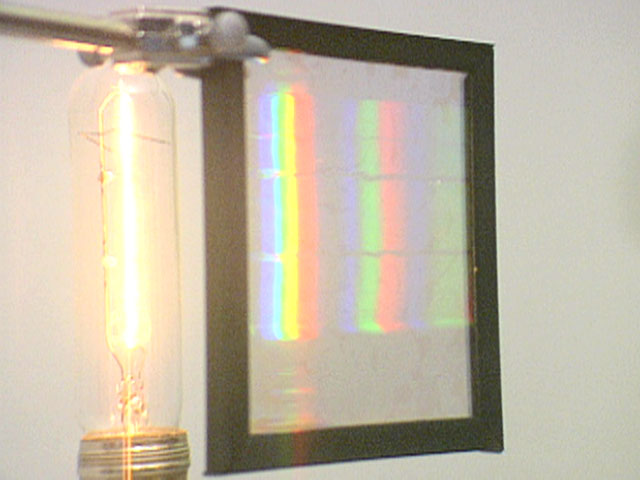
Two kinds of light sources are in common use, the incandescent bulb and the fluorescent light, the latter a modified mercury discharge lamp. Discussion In an incandescent bulb an electrical current passing through a tungsten filament heats it so that it glows white-hot. As you can see in the photo on the left, the spectrum of light from an incandescent light bulb is continuous. A mercury discharge lamp, such as that shown in the photo on the right, consists of an evacuated tube that contains a minute amount of mercury vapor and has an electrode at each end. When a high voltage, such as that produced by the power supply shown, is applied across the discharge lamp, electrons are ejected from the negative electrode, or cathode, and move rapidly toward the positive electrode, or anode. When these fast-moving electrons collide with a mercury atom, they can give it enough energy to raise, or "excite," one of its electrons from the ground, or lowest energy state, to a higher energy state. Such an excited atom is not stable, and releases energy in the form of photons. The spectrum of the light produced by such a discharge lamp is a line or discrete spectrum. Note that in these spectra only the lines in the visible region, from about 400 to 750 nm, can be seen. Spectral lines also exist in the ultraviolet and infrared regions, and are invisible to the human eye. As we have seen in the section "Standing Waves, Line Spectra, Resonance, and Energy Transfer," a continuous spectrum is produced when energy is absorbed or emitted by an unconfined wave, but a line or discrete spectrum is produced when energy is absorbed or emitted by a confined wave. Electromagnetic theory predicts it is electrons in a material that absorb or emit energy in the form of photons, so the continuous emission spectrum from an incandescent light bulb suggests that electrons in the metal lamp filament of an incandescent are unconfined waves, and the discrete emission spectrum from a discharge lamp suggests that electrons in gaseous atoms in the discharge lamp are confined waves. While this might seem obvious today, when Louis de Broglie (1892-1987), then a graduate student in physics at the University of Paris, submitted his doctoral thesis in 1924 proposing that matter, including electrons, had wave-like properties, it was scarcely believable. De Broglie's examining committee sent a copy of his thesis to Einstein, and only after Einstein had described it as brilliant did they award him his degree. Inspired by de Broglie's wave model of matter, the Austrian physicist Erwin Schrödinger (1887-1961) developed his wave or quantum mechanics in 1926. This new theory of mechanics predicted an emission spectrum for hydrogen atoms in complete agreement with the experimental spectrum, and is the basis for understanding the behavior of matter on an atomic scale. | |
| Table of Contents | Next Page |