|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
 |

View Slide Thumbnails |
|

View Next Movie |
|
Voiceover
A conductivity apparatus is placed in a beaker of deionized water and no light is observed. Placed in glacial acetic acid, no conductance is observed. When water is added to the pure acetic acid, the bulb glows brightly demonstrating the importance of water for the ionization of acetic acid.
Discussion
A conductivity apparatus is placed in a beaker of deionized water and no light is observed. When the conductivity apparatus is placed in glacial acetic acid no conductance is observed showing that water and glacial acetic acid are poor conductors of electricity (unionized). However, when water is added to the pure acetic acid, the bulb glows brightly demonstrating the importance of water for the ionization of acetic acid.
CH3CO2H(l) +
H2O(l)
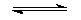 1
CH3CO2-(aq) + H3O+(aq) 1
CH3CO2-(aq) + H3O+(aq)
|
Design, Text and Demonstrator:
|
|
| |
Gary Trammell
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Videographer/Editor:
|
|
| |
Steve Dykema
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Voice:
|
|
| |
Margaret Biddle
|
University of Wisconsin - Madison, Madison, WI 53706
|
|
Audio Production:
|
|
| |
Greg Minix
|
University of Wisconsin - Madison, College of Engineering, Madison, WI 53706
|
| |
Jerrold J. Jacobsen
|
University of Wisconsin - Madison, Madison, WI 53706
|
|

