|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
| Bromination of Hexane Isomers |
 |

View Slide Thumbnails |
|

View Next Movie |
|
Voiceover
The isomeric hydrocarbons 2,3-dimethylbutane and 2,2-dimethylbutane are mixed with bromine and irradiated with a slide projector. 2,3-Dimethylbutane reacts more rapidly than the isomeric 2,2-dimethylbutane. This faster rate is attributed to the formation of the more stable tertiary radical intermediate. pH paper shows the presence of an acidic by-product.
Discussion
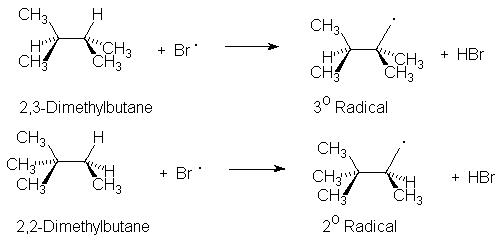
Bromination of Hexane Isomers |
|
|
|
|
|
Citation:
|
|
| |
Kolb, K. E.
|
Bradley University, unpublished results.
|
|
Design, Text and Demonstrator:
|
|
| |
Gary Trammell
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Videographer/Editor:
|
|
| |
Steve Dykema
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Voice:
|
|
| |
Margaret Biddle
|
University of Wisconsin - Madison, Madison, WI 53706
|
|
Audio Production:
|
|
| |
Greg Minix
|
University of Wisconsin - Madison, College of Engineering, Madison, WI 53706
|
| |
Jerrold J. Jacobsen
|
University of Wisconsin - Madison, Madison, WI 53706
|
|

