|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
| |
Click a thumbnail picture below to view a larger version. |

View Movie |
|
Narrative
Free rotation about the bond between carbons 2 and 3 of butane reveals steric crowding when the methyl groups are eclipsed. The energy decreases as the methyl groups move farther apart. The anti-conformation of butane, the lowest energy arrangement is formed when the methyl groups are opposite each other. This may be shown in a Newman projection. If the model is twisted (120°) a higher energy staggered conformation called the gauche form is obtained. The Newman projection of the gauche form of butane is illustrated.
Discussion
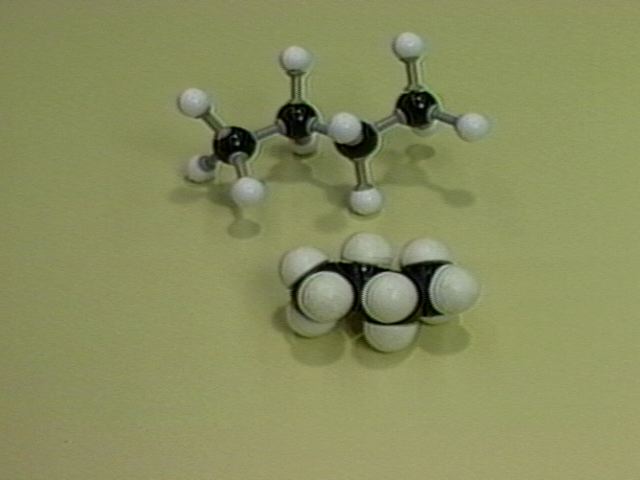
Ball-and-stick and Space-filling Models of Butane |
|
|
|
|
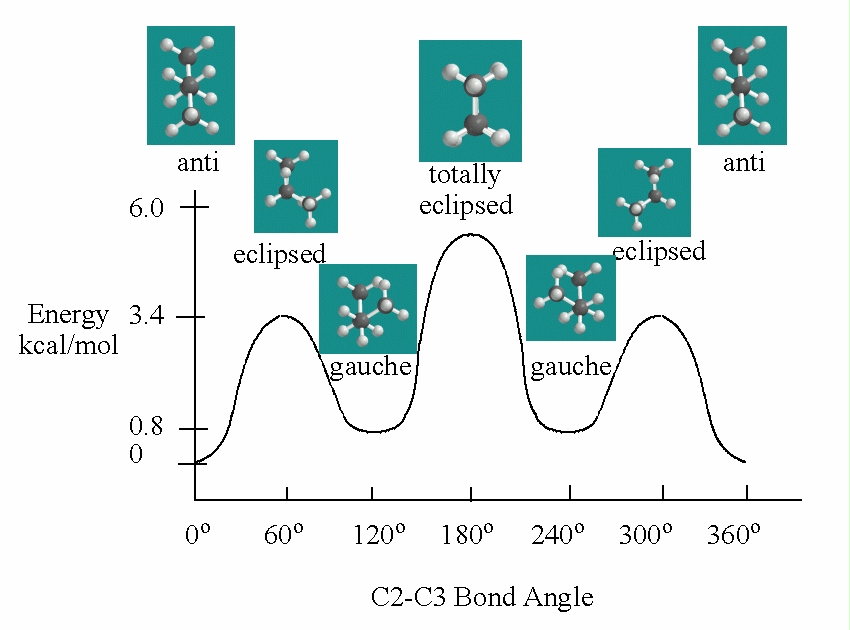
Energy versus Angle of Rotation for Butane |
|
|
|
|
|

