|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
 |

View Slide Thumbnails |
|

View Next Movie |
|
Voiceover
Free rotation about the carbon-carbon single bond of ethane is shown. In the eclipsed conformation, the distance between the adjacent hydrogen atoms is measured. If ethane is twisted into the staggered conformation, the distance between the adjacent hydrogen atoms increases. As the molecule is rotated about the carbon-carbon bond, notice how the conformations alternate between the staggered and eclipsed forms. This orientation is used to draw the Newman projection. A projection drawing of the head-on view of the staggered conformation of ethane is made. In this Newman projection the circle represents the carbon atom in the rear of the molecule. The center of the Y represents the carbon atom in the front of the molecule. If the molecule is twisted 60° into the eclipsed conformation, a second Newman projection is drawn.
Discussion
The pictures below show models of eclipsed ethane. The term "eclipsed" comes from the fact that in the Newman projection the front hydrogen atoms are between the viewer and the back hydrogen atoms. The front hydrogens eclipse the back ones just as the moon eclipses the sun in a solar eclipse.
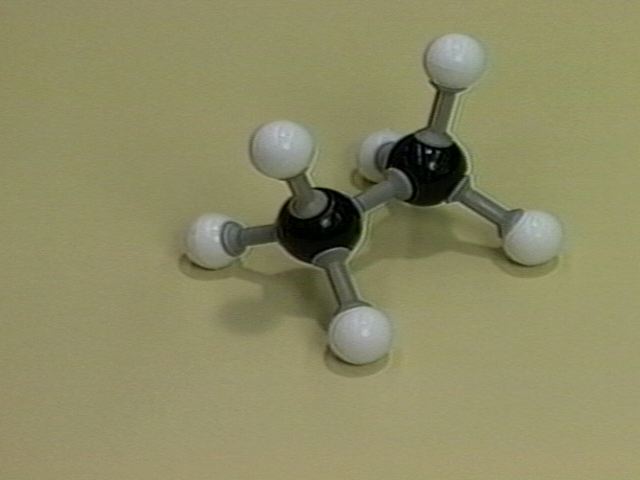
Ball-and-stick Model of Eclipsed Ethane |

Space-filling Model of Eclipsed Ethane |
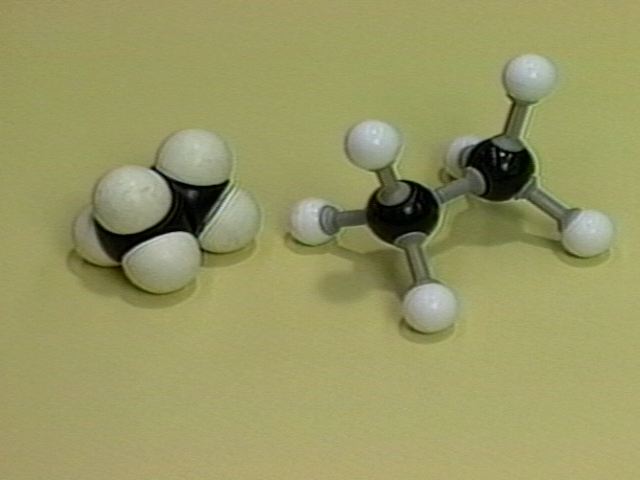
Ball-and-stick and Space-filling Models of Eclipsed Ethane |
|
|
|
Design, Text and Demonstrator:
|
|
| |
Gary Trammell
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Videographer/Editor:
|
|
| |
Steve Dykema
|
University of Illinois at Springfield, Springfield, IL 62794
|
|
Voice:
|
|
| |
Margaret Biddle
|
University of Wisconsin - Madison, Madison, WI 53706
|
|
Audio Production:
|
|
| |
Greg Minix
|
University of Wisconsin - Madison, College of Engineering, Madison, WI 53706
|
| |
Jerrold J. Jacobsen
|
University of Wisconsin - Madison, Madison, WI 53706
|
|

