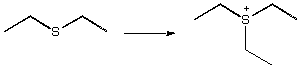
1067. Choose the reagent that would be best for these reactions.
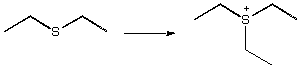
A. CH3CH2F
B. CH3CH2Br
C. CH3CH2C l
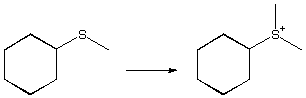
1068. Which reagents would not give the correct products for these reactions?
a.
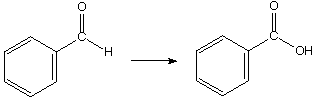
A. Ag2O, NH4OH
B. MeOH, HCl
C. CrO3, H2SO4
b.
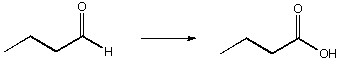
A. 1. K2Cr2O7, 2. H2SO4, H2O
B. CrO3, H2SO4
C. H2O
1069. Which reagents would give the right products for these reactions?
a.
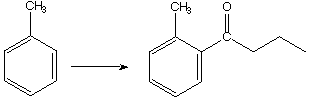
A. 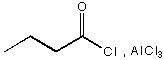
B. 1. 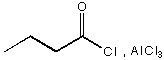 , 2. Zn(Hg), HC l
, 2. Zn(Hg), HC l
C.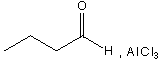
b.
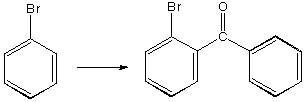
A.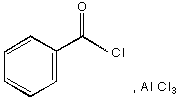
B. 1. 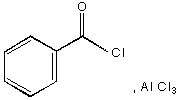 , 2. Zn(Hg), HC l
, 2. Zn(Hg), HC l
C. 
1070. Which reagents give the appropriate products for these reactions?
a.
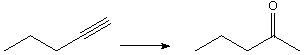
A. H2O
B. Hg(OAc)2 , NaBH4
C. H3O+ , Hg(OAc)2
D. 1. BH3, 2. NaOH, H2O2
b.
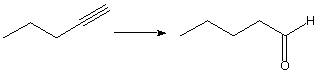
A. 1. BH3, 2. NaOH, H2O2
B. Hg(OAc)2, NaBH4
C. HgSO4, H3O+
D. H3O+, Hg(OAc)2
1126. Which of the following does NOT convert an alcohol into a good leaving group for a substitution reaction?
A. PBr3
B. HBr
C. Na
D. SOCl2
1127. A phase transfer catalyst is effective because
A. it dissolves one of the reagents
B. it dissolves both of the reagents
C. it dissolves in two immiscible solvents
D. it brings an ion into a nonpolar solvent
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..