
A. Cl2
B. Na
C. CH4
D. OsO4
1039. Which of the following alkynes could be used to make this ketone selectively?
A. 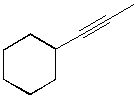 B.
B. 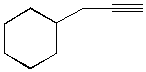
Which set of reagents would be appropriate for the transformation?
A. HgSO4, H2O
B. BH3 followed by H2O2 and NaOH
Click here to see this question used in the MultiStep format.
1045. This optically active cyclopentene undergoes photolytic bromination with N-bromosuccinimide.
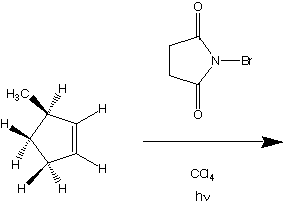
Which hydrogens can be abstracted?
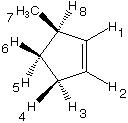
1, 2, 3, 4, 5, 6, 7, 8
Which hydrogen would be abstracted to form this radical?
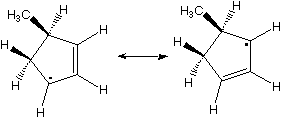
3,4,8
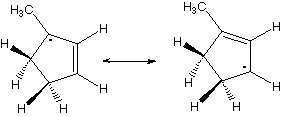
3,4,8
What are the products when hydrogens 3 and 4 are abstracted?
A.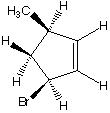 B.
B.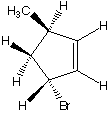 C.
C.
D.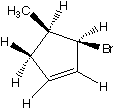 E.
E.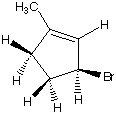 F.
F.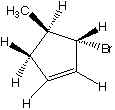
G.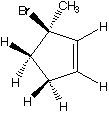 H.
H.
A, B, C, D, E, F, G, H
Would the products exhibit optical activity?
A. Yes B. No
What are the products when hydrogen 8 is abstracted?
A, B, C, D, E, F, G, H
Would these products exhibit optical activity?
A. Yes B. No
a.
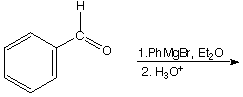
A. 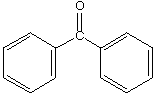 B.
B. 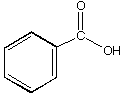
C. 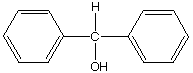 D.
D. 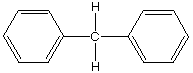
b.
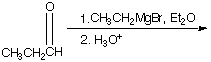
A. ![]() B.
B. 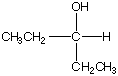
C. ![]() D.
D. 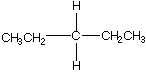
a.
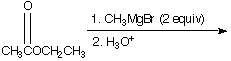
A. ![]() B.
B. 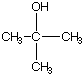 C.
C. 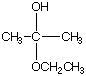
b.
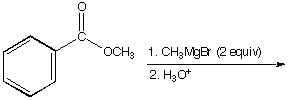
A. 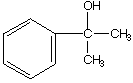 B.
B. 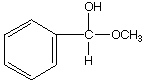 C.
C. ![]()
a.
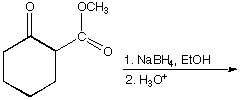
A. 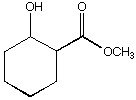 B.
B. 
C. 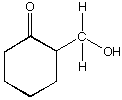 D.
D. 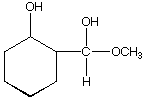
b.
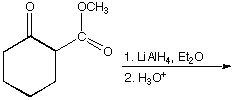
A.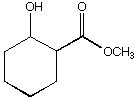 B.
B. 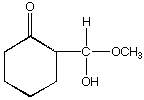
C. 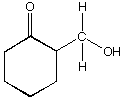 D.
D. 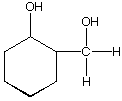
a.
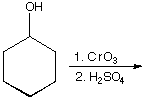
A. 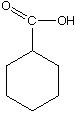 B.
B. 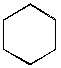 C.
C. 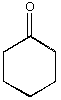
D. 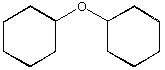
b.
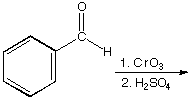
A. 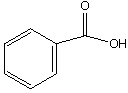 B.
B. 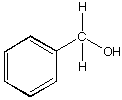
C. 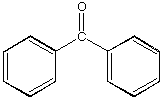 D.
D. 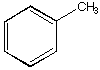
A. 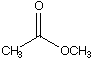 B.
B. 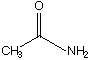 C.
C. 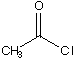
D. 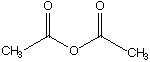
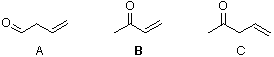
a. Which carbonyl compound is the least reactive? B
b. Which carbonyl compound is the most reactive? C
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..