Welcome to the Lipids debriefing.
Answer the questions in the spaces provided. If necessary, click on the Check buttons when you are finished with each problem.
|
1. On the paper structure of distearyl phosphatidyl choline shown below, click on the following groups: |
|||
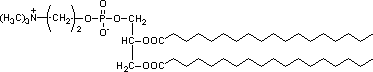
|
|
Glycerol |
Glycerol is the "scaffold" phospholipids are constructed around. |
|
Fatty acid(s) |
Fatty acids are long, linear hydrocarbons. |
||
|
|
Head Group |
The head group is polar and contains phosphorus. |
|
|
2. From the following molecules, check any that are amphiphiles. |
|||||
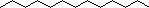 |
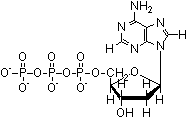 |
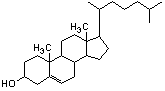 |
|||
|
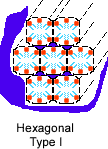
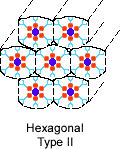
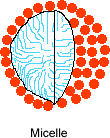
3. Which of the following structures do you think amphiphilic molecules could form in water? |
||||||
 |
 |
 |
 |
|||
| Hexagonal Type I | Hexagonal Type II | Micelle | ||||
|
|
Correct! The polar parts are on the outside of these two structures, so they can interact with water, which is also polar. The hydrophobic parts are kept away from water.
Correct! But is there another?
If water surrounds the structures, what part will it touch?
|
|||||
|
4. If the solvent were hexane instead, which of the structures above do you think would form? |
||||||
| Hexagonal Type I | Hexagonal Type II | Micelle | ||||
|
|
Correct! In the hydrophobic solvent hexane, the hydrophobic parts will orient themselves outward, burying the polar parts inside the cylinders. Any water that is present will reside there.
What parts, hydrophobic or polar, would hexane interact with?
|
|||||