
The Coordination Number: The coordination number of a metal atom in a hexagonal close-packed structure is 12. An atom touches three other atoms in the plane below it, six other atoms in the plane it is in, and three other atoms in the plane above it.
Number of Atoms in the Cell: A hexagonal close-packed metal has two atoms per unit cell. On average, there is one eighth of an atom at each corner of the cell and there is one atom within the cell.
The links on the right will show the coordination number and the number of cells in a HCP metal.
This is the end of the chapter: Unit Cells of Metals. Clicking any of the forward buttons or the Table of Contents button will return you to the main index page.
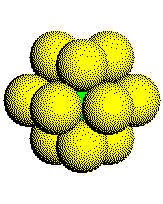
The coordination sphere of an atom in an HCP metal
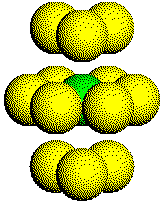
The expanded coordination sphere of an atom in an HCP metal
On average, one eighth of an atom is located inside the unit cell at each corner
One atom is located inside the unit cell

1 atom in the cell +
8 corners x 1/8 atom at each corner
= 2 atoms per cell
by: Journal of Chemical Education Software