
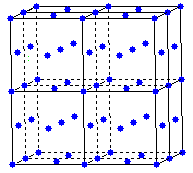
The sodium chloride structure is built on a face centered cubic space lattice.
Lattice points are placed at the centers of the anions (negative ions). Cations (positive ions) occupy all of the octahedral holes in the arrays.
NaCl-type unit cell contains four cations and four anions.
Click the links on the right to illustrate the topics listed above. The links above lead to definitions.

A
face centered cubic unit cell
A
face centered cubic lattice

Cations
occupy all of the octahedral holes in the face centered cubic array of
anions.

The NaCl - type structure crystallizes with a face centered cubic array of anions.

A
unit cell of the NaCl structure
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software