
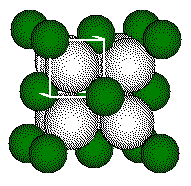
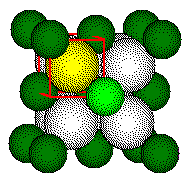
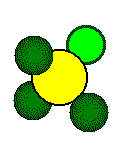
Calculations involving fluorite cell dimensions or ionic radii use the geometry of the small cube. The fluoride ion is at the center of the small cube and the fluoride ion and the calcium ion lie along the body diagonal of the small cube.
The distance between the center of
the fluoride ion and the center of the calcium ion is 1/2 the length of
the body diagonal of the small cube or 1/4 the length of the body diagonal
of the unit cell.
Click the links on the right to help illustrate these concepts.
x,
one half of the cell dimension of the CaF2 unit cell.
Cell
dimension of the CaF2 unit cell.
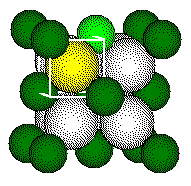
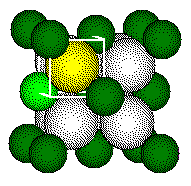
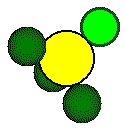
The
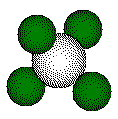
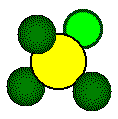
ions that form a tetrahedron lie on opposite corners of a cube.
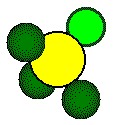
Each
highlighted calcium ion and highlighted fluoride touch along a body diagonal
of the cube.
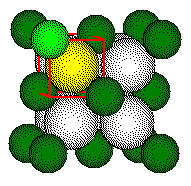
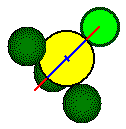
One
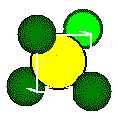
half of length of d, the body diagonal of the small cube, is equal to
the sum of the radius of the calcium ion and the radius of the fluoride
ion.
Body
diagonal
Ca2+
radius
F - radius
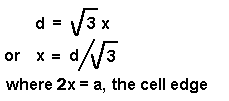
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software