
The fluorite unit cell contains a simple cubic array of fluoride ions.
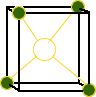
However the structure can not be described as a simple cubic because each fluoride ion does not have the same environment. The tetrahedra around the fluoride ions point in different directions.
Click on the image at right to view the two different tetrahedra.

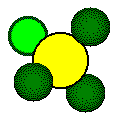
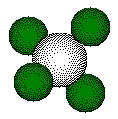
Calcium
fluoride crystallizes with a face centered cubic unit cell.
Fluoride
ions occupy all eight of the tetrahedral holes in the unit cell.
Click
on the picture to show the different tetrahedra.


Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software