Ionic Radii
Zn2+, 74 pm
S2-,
184 pm
Radius Ratio
= 74/184 = 0.40
Zinc blende is the cubic form of ZnS. There is also a hexaginal form that we will not discuss. The radius ratio is such that we expect the zinc ions to occupy tetrahedral holes in a close packed array of sulfide ions.
The stoichiometry (one Zn2+ ion per
S2- ion) requires that only one-half of the tetrahedral holes
be occupied.
Click on the layer of sulfide ions to the right to see the ZnS structure. The links above go to definitions.
Zn2+ Ion:
S2-:
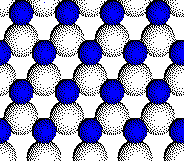
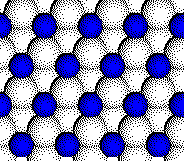
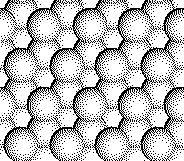

The
zinc blende (cubic ZnS) structure consists of a close packed array of
anions with half of the tetrahedral holes filled with cations.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software
.gif)