
In some structures all of the octahedral holes between two planes of anions are not filled. This page shows two arrangements of cations: one fills half of the holes, the other fills two-thirds of the holes.
The arrangement that fills half of the holes is found in the structures of TiO2 and NiF2. The arrangement that fills 2/3 of the holes is found in Al2O3 and Cr2O3.
Click the links on the right to view partially filled sets of octahedral holes.
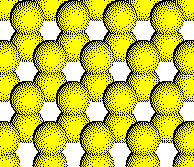
Anion
Layer 1
Anion
Layer 2
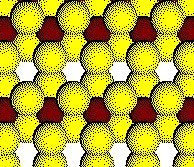
The octahedral holes are half filled.
Example: Rutile, TiO2
Example: Rutile, TiO2
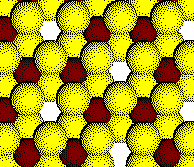
The octahedral holes are 2/3 filled.
Example: Alumina, Al2O3
Example: Alumina, Al2O3
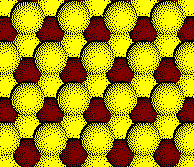
The octahedral holes are fully occupied.
Example: Cobalt Oxide, CoO
Example: Cobalt Oxide, CoO
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software