
We use the structure of NaCl to illustrate the structural features found in ionic compounds.

Na+
ion

Cl -
ion
1.
Ions of opposite charge touch so that the forces of attraction between
these ions are as large as possible.
2.
Each positive ion touches as many negative ions as possible in order to
maximize the forces of attraction.
3.
Positive ions do not touch other positive ions because they are small
and strongly repel each other.
4.
Negative ions can approach each other closely. In some structures, negative
ions touch other negative ions.
Click the link on the right to view the packing illustration.
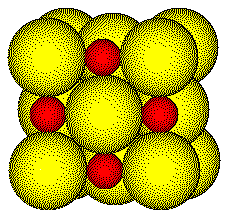
In
the NaCl structure, the chloride ions pack as effciently as possible and
the sodium ions occupy the left over space.
1.
Sodium ions touch chloride ions

2.
Sodium ions are surrounded by chloride ions
3.
Sodium ions do not touch other sodium ions
4.
Chloride ions approach each other closely
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software