
Laves' Principle:
The most stable structure for a solid is one in which the most efficient use is made of space. When a compound forms an ionic solid, its ions pack as closely as possible.
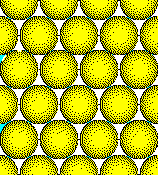
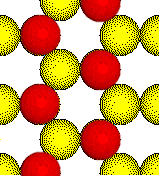
The ions in ionic solids fill space as
efficiently as possible.
Close packed arrays of anions are typical
in ionic solids.
This open arrangement of two different
ions is unknown in ionic solids.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software