Solving the Schrodinger wave equation results in a wave fuction (orbital) that describes the distribution of electron probability density in the vicinity of an atom nucleon (atomic orbital, A.O.) or a molecule (molecular orbital, M.O.).
Molecular orbitals can be approximated as linear combinations (weighted sums and differences) of atomic orbitals.
Several different atomic orbitals may be involved in the formation of a M.O. and each A.O. may contribute any fraction of its value to the combination.
Each molecular orbital is defined by a vector (set of coefficients c) in the M.O. wave function:
Where Á represents the M.O. and each Á, represents a different A.O.
The eigenvectors obtained by solved SCF (Self Consistent Field) wave functions to obtain the coefficients by which each atomic orbital is multiplied in the linear combination. In an eigenvector each coefficient reflects the relative contribution of a particular atomic orbital to the molecular orbital.
The Pauli Exclusion Principle states that the same eigenvector cannot describe mroe than two electrons. that is, each M.O. can accommodate no more than two electrons.
The contribution of an A.O. to the electron density of a molecular orbital depends on the square of its coefficient. Because of this. the sign of the coefficient is not important, but the size of the coefficient is very important.
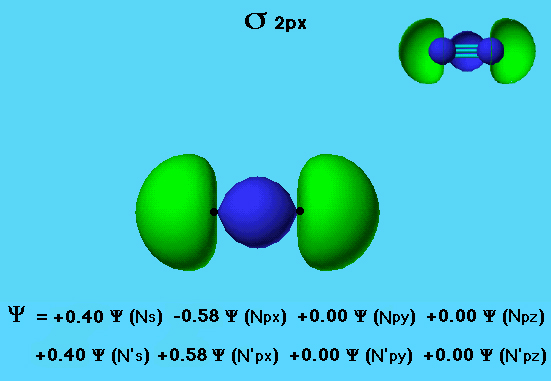
Nitrogen - sigma 2px
The σ 2px orbital in the nitrogen molecule includes considerable mixing of the s and p orbitals. The eigenvector coefficients show that the majority of the electron density comes from the 2px atomic orbitals (0.58) with significant contribution from the 2s atomic orbitals (0.40).
Nitric Oxide - sigma 2px
The σ 2px orbital in the nitric oxide molecule shows considerable mixing of the s and p orbitals for the nitrogen atom, but not for the more electronegative oxygen atom. The eigenvector coefficients show that the electron density comes about equally from the 2s (0.69) and 2px (-0.63) atomic orbitals of nitrogen, with only a small contribution from the 2px atomic orbital (0.34) and a very small contribution from the 2s atomic orbital (0.05) of oxygen.

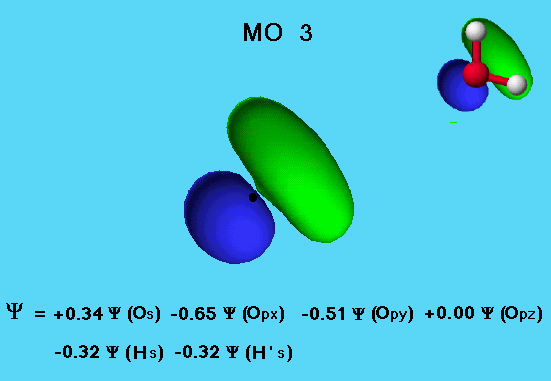
Water - MO3
The MO 3 orbital in the water molecule shows considerable mixing of the s and p orbitals of the oxygen atom. The eigenvector coefficients show that the majority of the electron density comes about equally from the 2px and 2py atomic orbitals on oxygen, with a minor contribution from the 2s atomic orbital of oxygen. The hydrogen 1s orbitals are also participating in the bonding.
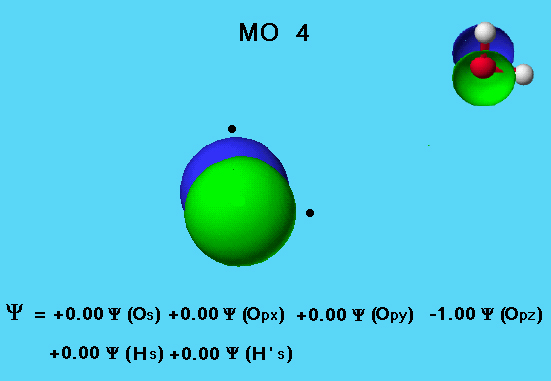
Water - MO 4
The MO 4 (nonbonding 2 pz) orbital in the water molecule shows no mixing of the s and p orbitals. The eigenvector coefficients show that all of the electron density comes from the oxygen 2pz atomic orbital. The hydrogen 1s orbitals do not participate in this molecular orbital. This is because the hydrogen 1s orbitals overlap equally with positive and negative lopes of the oxygen 2pz wave function and the total overlap sums to zero.