A molecule or ion exhibits resonance when two or more acceptable Lewis diagrams can be drawn to describe the bonding for the same arrangement of atoms. The true distribution of electrons is thought to be an average of all the diagrams.
Atoms
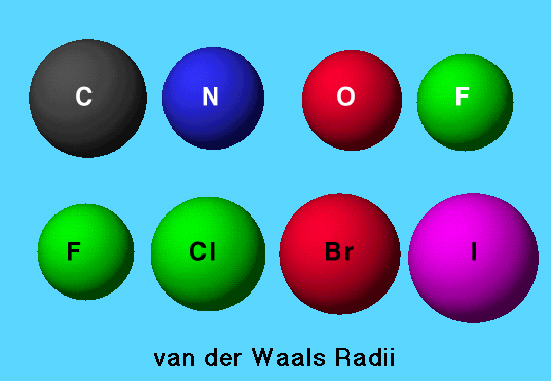
The graphic shows the typical periodic variation of sizes of the van der Waals radii for atoms in the same period and for atoms in the same group.
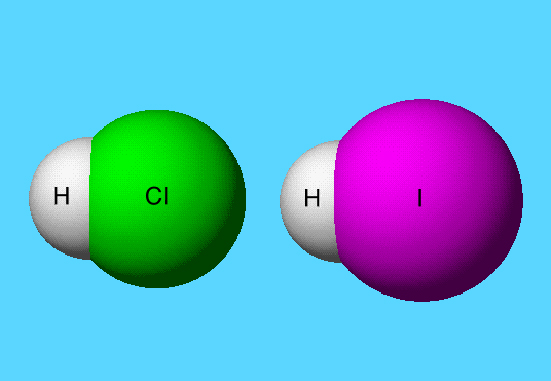
HCl
The graphic shows that the closest approach of a H atom to a Cl atom is 2.97 Å. The graphic also shows the covalent bonded distance is 1.27 Å, and shows the overlapping of van der Waals radii of H and Cl atoms within the same molecule.

HCl and HI
A model of a molecule that uses van der Waals radii for atom spheres is known as a space-filling model since it approximately represents the outer electron density of the molecule.