Quantum mechanical calculations generate values for partial charges for the atoms in a molecule. These are related to electron densities around various atoms resulting from bonding and lone pairs of electrons. If no graphic for partial charge is given for a molecule in this database, the partial charges on atoms are roughly equal or are very small.
The relative scales are not the same for partial charges on atoms of different molecules.
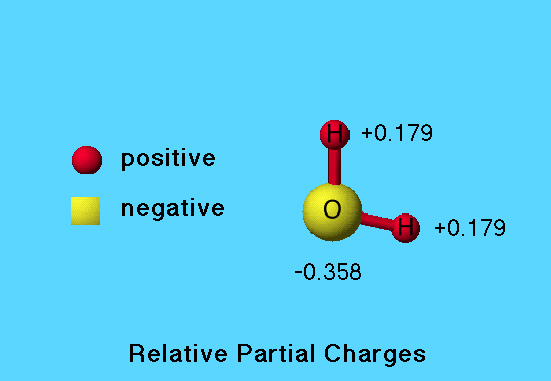
Partial Charges
In this model of the water molecule, relative size of the sphere corresponds to the magnitude of the partial charge; the larger the sphere, the greater the partial charge. Positive partial charge is colored red and negative partial charge is colored yellow. In this case oxygen has a partial charge of -0.358, and each hydrogen has a partial charge of +0.179. For a neutral molecule the sum of partial charges over all atoms is always zero.
Electronegativity
A very rough approximation of partial charges results from a qualitative consideration of electronegativities. The atom with the higher electronegativity is partially negative, the atom with the lower electronegativity is partially positive.

Water Dipole
The visualization of the partial charge may be useful in determining the direction of the dipole moment. The net dipole vector is drawn from the region of the molecule that has a partial positive charge toward the region of the molecule that has a partial negative charge.
