The mathematical solutions of the wave functions that define an atomic or molecular orbital may have either a positive or negative sign. A given wave function may have a single sign or may have both positive and negative phases. For example, a 1s orbital has a single sign but a 2p orbital has two—one lobe of the orbital results from the positive phase and the other from the negative phase of the wave function. The wave function is squared to obtain the probability density, thus the value of the probability density is always positive, regardless of the sign of the wave function. The sign of the wave function for each orbital (or orbital lobe) is indicated here by color.
Occupied orbitals are colored blue and green. Unoccupied orbitals are red and yellow. Color assignment with respect to the positive and negative phases of the wave function is arbitrary, so identical orbitals may be colored differently in different graphics.
(That is, in some occupied M.O.’s blue represents positive phase, but in others blue represents negative phase. While this may seem very confusing, please remember that we usually are not interested in the sign or phase of a particular orbital or lobe; we only need to know if lobes in an orbital have the same or different signs.)
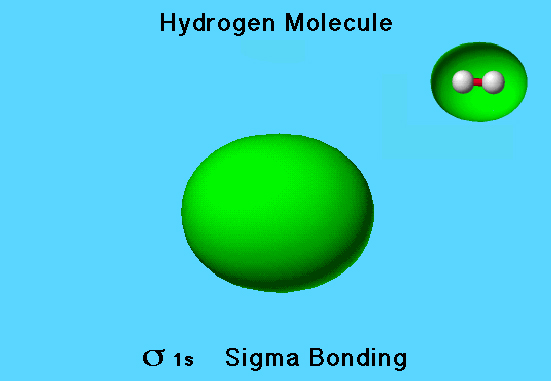
Hydrogen Molecule Bonding MO
The hydrogen molecule has a filled σ 1s (sigma bonding) molecular orbital that is assigned the color green. The sign is the same everywhere in this orbital.
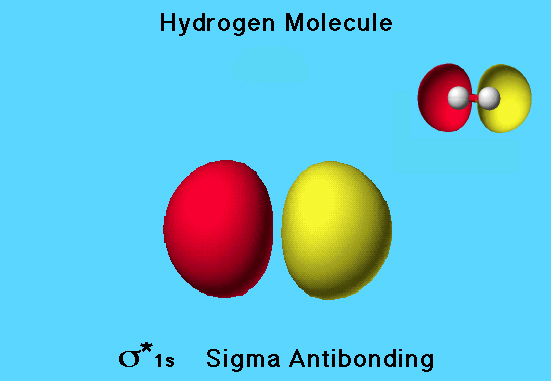
Hydrogen Molecule Antibonding MO
The hydrogen molecule has a σ* 1s (sigma antibonding) molecular orbital that is unoccupied. Because the sign of the wave function for one side of the orbital is opposite to the sign in the other side, one side of the orbital is colored red and the other is colored yellow.
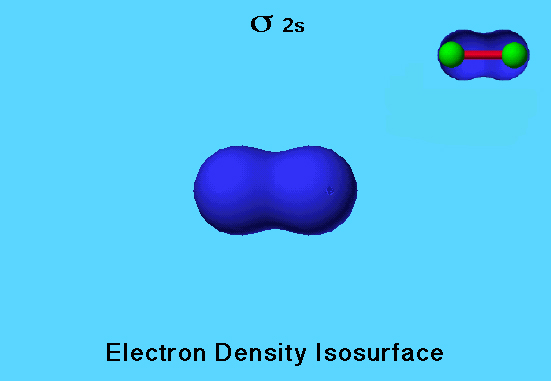
Flourine Molecule Bonding MO
The fluorine molecule has a filled σ 2s (sigma bonding) orbital that is colored blue because on the isocontour surface, all parts of the wave function have the same sign. There are three other bonding orbitals in fluorine that are occupied.
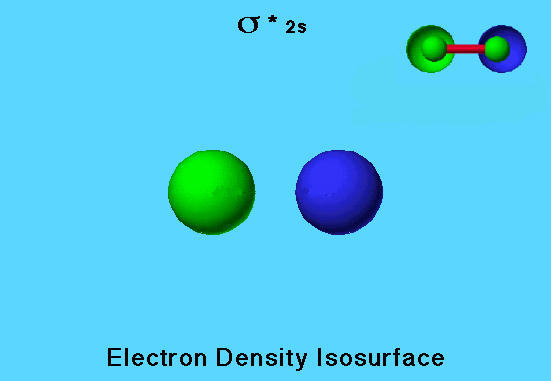
Flourine Molecule Antibonding MO
The fluorine molecule has a filled <sigma>* 2s (sigma antibonding) molecular orbital. Because the sign of the wave function for one side of the orbital is opposite to the sign in the other side, one side of the orbital is colored green and the other is colored blue. There are two other antibonding MO’s in fluorine that are occupied, and one that is not occupied.