X-ray crystallography and other experimental techniques tell us where the atoms are located in a molecule. We define molecular geometry as the positions of the atomic nuclei in a molecule. According to VSEPR theory, molecular geometry can be predicted by starting with the electron pair geometry about the central atom and adding atoms to some or all of the electron pairs. This model produces good agreement with experimental determinations for simple molecules.
Methane
Methane has four bonded H atoms around the C atom with no lone pairs of electrons. Its molecular geometry is a tetrahedron.

Ammonia
Ammonia has three bonded H atoms and one lone pair of electrons around the N atom. Its molecular geometry is a trigonal pyramid.

Water
Water has two bonded H atoms and two lone pairs of electrons around the O atom. Its molecular geometry is bent, or V-shaped.

Hydrogen Fluoride
Hydrogen fluoride has one bonded H atom and three lone pairs of electrons around the F atom. Its molecular geometry is linear. (All two-atom molecules are linear, regardless of the number of electron pairs about either atom.)

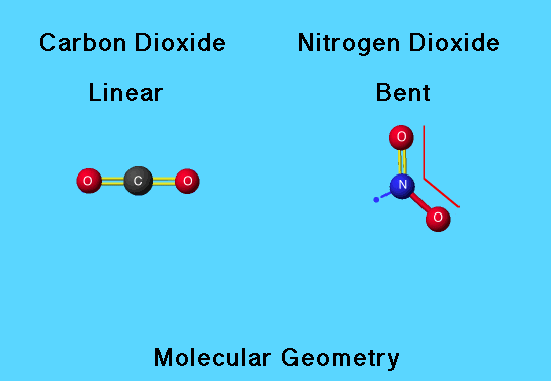
Triatomics
Carbon dioxide has both linear electron pair and molecular geometry since there are two carbon-oxygen double bonds (each counting as one pair) and no lone pairs about the central C atom.
For nitrogen dioxide, the lone nonbonded electron occupies a position equivalent in the trigonal planar electron-pair geometry. This gives rise to a bent or V-shaped molecular geometry. The single nonbonded electron exerts less repulsion than a bond or a pair of nonbonded electrons would, allowing the bond to be wider than the 120° predicted by the trigonal planar electron-pair geometry. The O—N—O bond angle in nitrogen dioxide is 134.1°.
Tetraatomics
Sulfur trioxide, with three sulfur-oxygen bonds and no lone pairs of electrons on the sulfur, has trigonal planar electron pair and molecular geometry. The sulfite ion has three sulfur-oxygen bonds and one lone pair to give the tetrahedral electron pair geometry a trigonal pyramidal molecular geometry.
