According to molecular orbital theory, electrons in a molecule exist in orbitals that are characteristic of the molecule as a whole. Molecular orbitals in their simplest approximate form are considered to be linear combinations (sums and differences) of the wave functions that define atomic orbitals. A combination of waves can be constructive, producing a larger wave (area of high electron density), or destructive producing a smaller wave or a node (area of low or no electron density).
The number of molecular orbitals formed is equal to the number of atomic orbitals combined. That is, two atomic orbitals always combine to form two molecular orbitals. Like atomic orbitals, each molecular orbital can hold a maximum of two electrons.
Molecular orbitals are classified as bonding, antibonding, or nonbonding. Bonding orbitals generally have lower energy than the corresponding atomic orbitals. Through constructive overlap they concentrate electron density between atomic nuclei. Antibonding orbitals generally have higher energy than the corresponding atomic orbitals. Through destructive overlap they have very low electron density between nuclei. Nonbonding orbitals correspond to no net overlap of atomic orbitals.
The molecular orbital visualizations in this database have been produced by the CAChe Scientific MOPAC PM3 semi-empirical quantum mechanical computations.
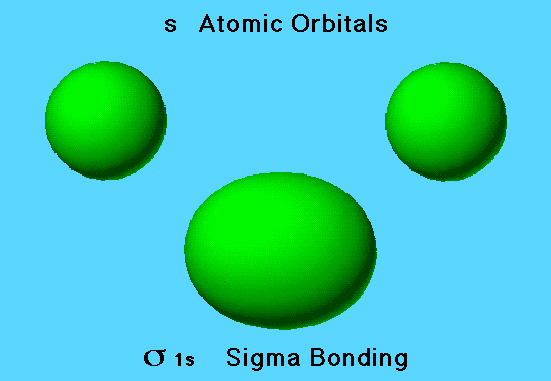
Bonding MO in Hydrogen Molecule
As two hydrogen atoms approach each other from a distance, the electron densities from the 1s orbitals overlap. If electron densities overlap constructively forming areas of high electron density between the nuclei, the result is a σ 1s (sigma bonding) orbital. This corresponds to an addition of wave functions, for this example.
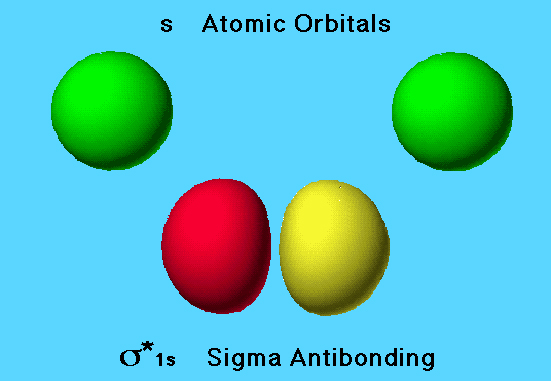
Antibonding MO in Hydrogen Molecule
Another combination of the same atomic orbitals is possible, corresponding to subtraction of wave functions for this example. The net effect of this operation results in very little electron density between the nuclei. As the atoms get closer together, the electron densities overlap destructively, forming areas of low electron density between the nuclei. The orbital formed is referred to as a σ* 1s (sigma antibonding) orbital.