All molecules have a HOMO (highest occupied molecular orbital) and a LUMO (lowest unoccupied molecular orbital).
It is often through overlap of the HOMO of one molecule with the LUMO of another that new bonds are formed during chemical reactions.
In order for bonds to form, the overlap of the orbitals must be constructive. In the regions of large overlap the orbitals (wave functions) must have the same sign.
In this program, color codes for the wave function signs are arbitrary. Constructive overlap is possible between the blue region of an occupied orbital and the yellow region of an unoccupied orbital or between the green region of an occupied and the red region of an unoccupied orbital.
If two constructive interactions are possible, the one with the lower energy difference between the HOMO and LUMO is more likely to occur.
In order to compare the HOMO of one molecule with the LUMO of another, select M.O. Reaction Predict from the Optional Views menu. Then, by selecting the HOMO/LUMO option in the Compare mode, the database will display the HOMO of the currently selected molecule with the LUMO of the molecule chosen from the molecule comparison list.
Molecular Orbitals
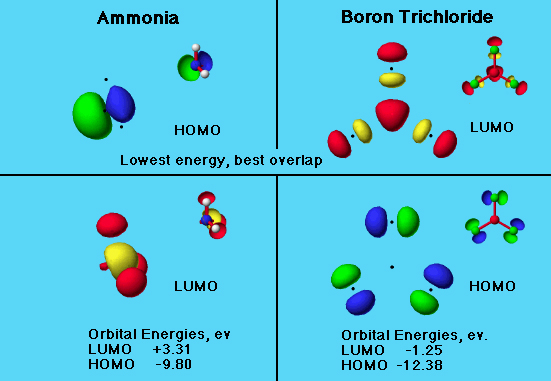
The graphic shows the MO's as seen using the Compare feature in the database.
A check for the smaller energy differences of these orbitals is needed. Find the energy difference between the LUMO of one and the HOMO of the other and vice versa.
Ammonia Boron trichloride
LUMO +3.31 -1.25
HOMO -9.80 -12.38
The smaller energy difference is between the ammonia HOMO and boron trichloride LUMO. A visual inspection of the orbitals shows that the nitrogen atom of the ammonia molecule were to approach the boron atom in boron trichloride, there would be constructive (green-red) overlap.
Reaction Prediction
This graphic shows the HOMO and LUMO with their front surfaces cut away to reveal the positions of the ball and stick models. Notice that the LUMO of the boron trichloride molecule overlaps constructively (red-green) with the HOMO of the ammonia molecule.
Thus the predicted reaction is a sharing of electrons from the HOMO of the ammonia molecule with the LUMO of boron trichloride. This is a description of a Lewis acid/base reaction. Ammonia, a Lewis base, shares electrons from its HOMO with the LUMO of boron trichloride, a Lewis acid.
