Linus Pauling first defined electronegativity as: “The power of an atom in a molecule to attract electrons to itself.” His original electronegativity scale was based upon thermochemical data that show that a bond between two different atoms A—B is stronger than the average of the A—A and B—B bonds. Updated Pauling electronegativity values are from J. Inorg. Nucl. Chem., 17, 215 (1961).
The greater the electronegativity difference between atoms in a bond, the more polar the bond.
HCl Electronegativity
Pauling set up the electronegativity scale relative to hydrogen, which was assigned a value of 2.2. Chlorine has an electronegativity of 3.16 on this scale.

HCl Partial Charge
Electronegativity values are used in a qualitative sense to predict separation of electric charges in a molecule. In a bonded pair of atoms, the atom with higher electronegativity is partially negative, while the atom with a lower electronegativity is partially positive. A separation of partial charges like this is called a dipole and the bond between the atoms is called a polar bond.

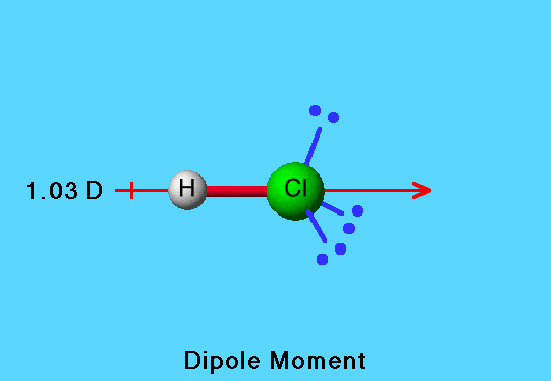
HCl Dipole
Electronegativity values are also used to predict the direction of bond dipoles. The molecular dipole depends on both bond dipoles and the effects of lone pairs. In HCl, the negative end (arrowhead) of the dipole vector is in the direction of chlorine, the atom of higher electronegativity and the atom that has lone pairs of electrons.