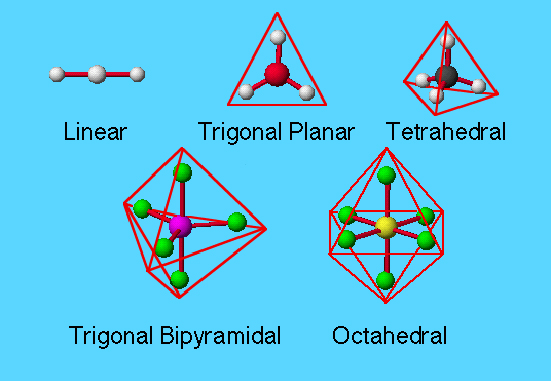
The shape of a molecule can be predicted based on the number and arrangement of electron pairs around a central atom. The geometry is determined by minimizing the repulsions between electron pairs in the bonds between atoms and/or lone pairs of electrons as postulated by VSEPR (Valence Shell Electron Pair Repulsion) model.
Electron Pair Geometry
The geometry types are defined by the number of electron pairs around an atom. Electron pairs are defined as electrons in bonds, lone pairs of electrons, and occasionally a single unpaired electron.
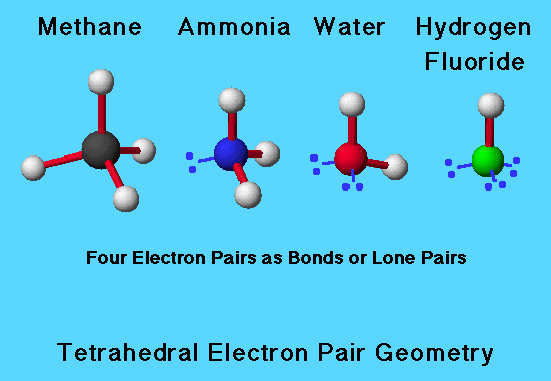
Compare Tetrahedra
In methane, ammonia, water, and hydrogen fluoride, the electron pair geometry is tetrahedral. All have four pairs of electrons about the central atom (C, N, O, or F). In methane, C has four bonds; in ammonia, N has 3 bonds and one lone pair; in water, O has 2 bonds and 2 lone pairs of electrons; and in hydrogen fluoride, F has 1 bond and 3 lone pairs.
Carbon Dioxide
Double or triple bonds count as “one pair” of electrons for the purpose of establishing the electron pair geometry. In carbon dioxide, the two double bonds count as two pairs of electrons around the carbon atom, predicting a linear geometry.
