Electrons in a polar covalent bond are unequally shared between the two bonded atoms, which results in partial positive and negative charges. The separation of the partial charges creates a dipole. The word dipole means two poles: the separated partial positive and negative charges. A polar molecule results when a molecule contains polar bonds in an unsymmetrical arrangement.
Nonpolar molecules are of two types. Molecules whose atoms have equal or nearly equal electronegativities have zero or very small dipole moments. A second type of nonpolar molecule has polar bonds, but the molecular geometry is symmetrical allowing the bond dipoles to cancel each other.
The dipole moments listed are experimental values. If a dipole moment was not available, it is designated with n. a. (not available).
Electronegativity
A very rough approximation of partial charges results from a qualitative consideration of electronegativity. The atom with the higher electronegativity is partially negative. The atom with the lower electronegativity is partially positive.

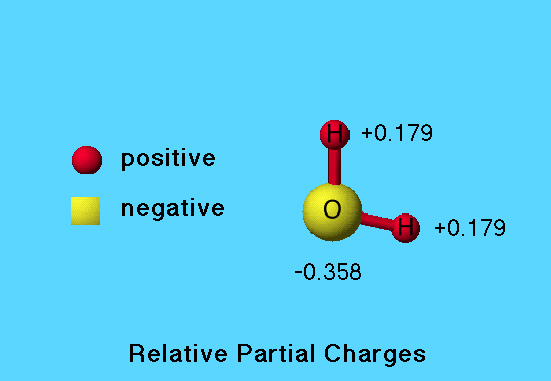
Partial Charge
In this model of the water molecule, relative size of the sphere corresponds to the magnitude of the partial charge; the larger the sphere, the greater the partial charge.
The relative scares are not the same for partial charges on atoms of different molecules.
Positive partial charge is colored red and negative partial charge is colored yellow. In this case oxygen has a partial charge of -0.358, and each hydrogen has a partial charge of +0.179. For a neutral molecule the sum of partial charges over all atoms is always zero.
Water Dipole
The polarity of a molecule is indicated by the dipole moment. the dipole moment, measured in debye units, is defined as the product of the distance separating charges of equal magnitude and opposite sign and the magnitude of the charge. Each polar bond has a dipole moment vector. If there are no lone pairs of electrons the dipole moment of a melecule is the sum of the bond dipole vectors drawn from the partial positive charge. If there are lone pairs, they also contribute to the dipole moment. The dipole moment arrow in the graphic for each polar molecule is an approximation of its direction.

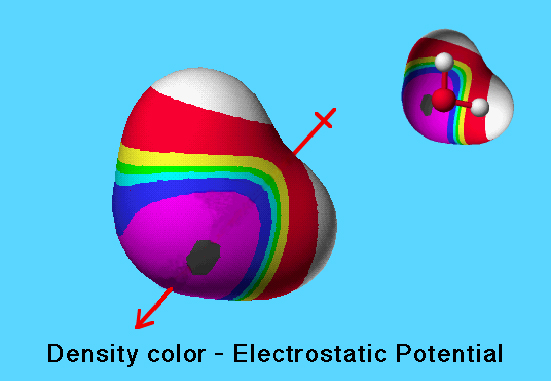
Electrostatic Potential
The electrostatic potential values are shown on the electron density volume isosurface using the spectral ordering of colors to represent electrostatic potential values: white (most positive; proton most strongly repelled) > red > yellow > green > cyan > blue > violet > charcoal (most negative; proton most strongly attracted).
Regions of low electron density are colored white or red to indicate positive electrostatic potential; regions of high electron density are colored blue or violet to indicate negative electrostatic potential.