Bond length is the distance between nuclei of two bonded atoms in a molecule. The bond lengths reported here correspond to the best available experimental values obtained from various literature sources listed on the reference page. If no literature date were available, bond lengths were calculated using PM3 MOPAC; these are designated with an asterisk (*).
Bond lengths are drawn to the same scale in each molecule so that visual comparisons may be made.
Compare Bond Length
The bond length in a molecule is determined by attractive forces between electrons and nuclei and repulsive forces between electrons or two nuclei. As one atom approaches another, constructive interference between wave functions concentrates electron probability density between the nuclei. This increased electron density attracts the nuclei together, lowering the total energy of the two-atom system. However, when the nuclei get too close together, the nucleus-nucleus repulsion and repulsions between electrons crowded between the nuclei begin to counteract the attractive forces. The bond length represents a minimum energy for the system. If the nuclei are closer than the bond length, repulsions cause the energy to be higher; if the internuclear distance is greater than the bond length, attractions are weaker and the energy is higher.

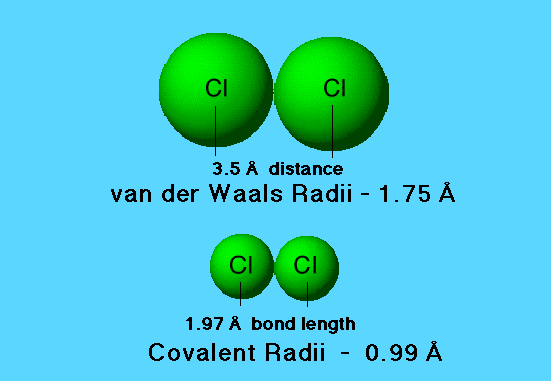
Chlorine
The bond length for chlorine is less than the sum of the van der Waals radii. The difference arises because there is increased electron density in the region between the nuclei. The additional electron density attracts the nuclei toward one another.
Bond Types
Bond length is influenced by bond order (triple, double, and single bonds). The lower the bond order, the longer the bond length as observed in the series of nitrogen, oxygen, and fluorine molecules.
