A Bond angle is defined as the angle between two bonds connecting three atoms. The bond angles of the molecules in this database correspond with the best available experimental values obtained from various literature sources listed on the reference page. A few angles for which literature values were unavailable were calculated using PM3 Mopac. These angles are marked with an asterisk (*).
Ideal Angles
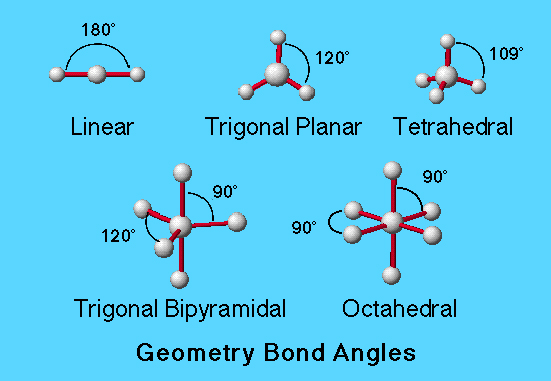
Bond angles in common molecular geometries:
Trigonal Planar................120 º
Tetrahedral......................109.5 º
Trigonal Bipyramidal......120 º, 90 º
Octahedral.......................90 º
Deviations
Deviations from the ideal bond angle of 109.5 º in molecules with tetrahedral geometries can be explained if we assume that lone-pair electron clouds are “fatter” than bond-pair electrons. This makes sense because a bond pair is attracted by two nuclei (one on each end of the bond) and therefore is localized between the nuclei. Fatter pairs repel other lone pairs more strongly than they repel bonded pairs. Lone-pair/bond pair repulsions are larger than bond-pair/bond-pair repulsions.
In the water molecule, the largest repulsion is between the two lone pairs, and the smallest is between the two bond pairs. If the bond angle decreases slightly from the ideal value of 109.5 º, all repulsions can be equalized. The experimental bond angle in water is 104.4 º. Ammonia, which has one pair of non-bonded electrons, has bond angles of 107.3 º, intermediate between those found in water and in the ideal tetrahedral structure.

Dihedral Angles
A dihedral angle is the angle between two intersecting places. Four points can define such planes. In the case of a molecule, a dihedral angle is useful for defining rotation around a bond, and so the molecule must have four atoms arranged in the pattern 1-2-3-4. A simple example of such a molecule is H-O-O-H, hydrogen peroxide. For a molecule shaped like ammonia, with three atoms bonded to a single central atom, the concept of a dihedral angle is much less useful.
In the hydrogen peroxide molecule shown, the dihedral angle defines how far one of the O-H bonds has rotated around the O-O bond, relative to the other O-H bond. It is easiest to see when the molecule is viewed from the end, with the oxygen atoms (labeled 2 and 3) aligned. From this point of view, the two oxygen atoms and the bond between look like a single atom. The dihedral angle is what appears to be an H-O-H angle. With the hydrogen atoms labeled 1 and 4, the angle is actually the angle between the planes defined by atoms 1, 2, and 3 and the plane defined by atoms 2, 3, and 4.
