In this model, the atoms are depicted as spheres (balls) and bonds as rods
(sticks). Each atom's radius is arbitrarily set at 20% of the van der Waals
radius. Unbonded electrons are shown as blue dots with lines indicating
their orientation in space. Atoms are colored by element: red for oxygen,
blue for nitrogen, gray for carbon, white for hydrogen, yellow for sulfur,
green for chlorine, purple for iodine, etc.
Atom Scale
Relative atomic radii are illustrated here with hydrogen, chlorine, and iodine.
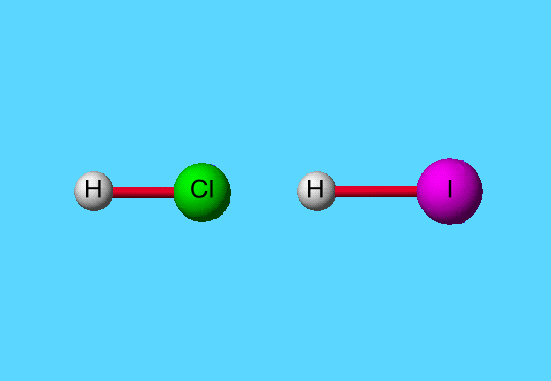
Bond Types
Each bond rod represents a pair of electrons. The bonds are color coded as follows:
- red for single bonds (a single rod or stick)
- yellow for double bonds (two rods or sticks)
- cyan for triple bonds (three rods or sticks)

Electron Pairs
Bonded atoms are located at distances and angles that
have been obtained from the chemical literature. Sources of data are
listed on the reference card at the end of the program. The nonbonded
electron pairs have been manually located in approximately correct
positions.
