 |
|
|
|
|
|
||||||||||||||||||||||||||||
Introduction to STMErnie Frank
|
||||||||||||||||||||||||||||||
|
|
| Figure 1. A block diagram of an STM. The Bias Control is used to adjust the voltage between the tip and sample. The Feedback Control compares the tunneling current observed to the desired current to be maintained (the Set Current) and sends out a correction voltage to the Piezo Amplifier which adjusts the tip/sample separation. This correction voltage is also sent to a computer and stored there. This data provides topographic information about the surface and is used to make a visual image. The X and Y controls sweep (raster) the tip across the area of the surface studied. |
The mechanical operation of an STM instrument is similar to a phonograph record player. On a record player, a needle is moved through a groove on the record. The needle bounces over bumps in the groove. The needle is connected to a device that converts the movement of the needle into a voltage. The voltage is then amplified and played as an audible sound. An STM instrument is basically an "atomic record player." A sharp tip is positioned close enough to a surface that electrons can tunnel between the sample and tip. The tip sweeps, or rasters, back and forth over the "bumps". In the case of the STM, the bumps are atoms. Unlike a record player where the needle is in physical contact with the record, in an STM the tip remains a fixed distance above the surface. This distance is maintained with a feedback control system that constantly monitors and corrects for any change in the tunneling current by adjusting the tip-sample separation (see Figure 1). A computer then records the amount the tip must change height to maintain a constant tunneling current. This is recorded as the STM image. Therefore, by accurately monitoring the tunneling current (which is exponentially related to the tip/sample separation) one can map out the changes in height on the sample surface and can thus obtain a topographic image of the surface.
An extremely sharp tip is required to obtain a good STM image--a single-atom tip. Just as a dull phonograph needle makes the music sound fuzzy, a dull STM tip makes the STM images appear fuzzy. For STM, a sharp single-atom tip is required to insure that electrons tunnel to or from a single point of the tip. If electrons can tunnel from multiple atoms or points on the tip, the resolution of the image is poor. To understand why, try to trace the features of a rough object with a needle. Repeat the procedure with a tennis ball. The needle traces along all the fine features of the surface, but the ball rolls over the surface obscuring most of the smaller features. Single-atom tips can be made either a "high tech" way (for instance using ion beams to machine sharp tips) or a "low tech" way (a piece of wire and a pair of wire cutters often works). No matter how it is done, the tip must have a single stable atom at the very tip.
Positioning the tip and keeping it within 10 angstroms of a surface is also difficult, but not impossible. It is complicated by the fact that it necessary not only to position the tip to within 10 angstroms, but also at times to position it several centimeters away from the surface. This long range of motion is necessary because the sample and tip need to be changed periodically and it is nearly impossible to do such a manipulation of two objects that are so very together without their bumping into each other. (Remember, a dull tip is bad for STM, and nothing dulls a tip like crashing it into something!) The long-range movement and positioning is usually performed mechanically. The most common positioners are a lever/fulcrum assembly with a large mechanical advantage or with an inertial "slip-stick" device as shown in Figure 2.
|
|
| Figure 2. Diagram of a Slip-Stick walker. |
Once you get the tip within 10 angstroms of the surface and start measuring tunneling current, you need to keep the separation distance constant as you sweep the tip across the surface. This is accomplished with a piezoelectric device (see Figure 3). Piezoelectric materials will expand and contract under an applied voltage. These materials are really not as exotic as you may think. Quartz is a common piezo material and is used in watches and clocks. Piezo devices are also commonly used in stereo speakers, where an applied voltage causes the piezo to move, which moves the speaker element making the sound. Mounting the tip on the end of a piezo device makes highly accurate and precise positioning of the tip possible (adjustments of less than 0.01 angstroms) by applying the appropriate voltage to the piezo. By recording the voltage applied to the piezo necessary to maintain a constant tunneling current, and therefore a constant tip-sample separation, topographic information of the surface is obtained and displayed as an image.
 |
| Figure 3. Cryogenic Scanning Tunneling Microscope (CSTM). The tip is mounted on the end of a piezo tube scanner, a sectioned piezo device capable of maintaining an accurate tip-sample separation based on tunneling current while rastering the tip across the area of the surface. Samples to be imaged are positioned on the copper sample stage. The microscope is suspended within a vacuum chamber on springs to vibrationally isolate the apparatus. The Burleigh Inchworm walker moves the tip far away when samples or tips are transferred. Diagram by Harry Chen, University of Wisconsin-Madison. |
Once the tip is positioned above the surface, extra caution must be taken to minimize vibrations. Any unwanted vibration may cause changes in the tip-sample separation producing unwanted signals, called noise, in the STM image. Larger vibrations may even cause the tip to crash into the sample. Most STM instruments either hang from springs, float on an air isolation table, or both in an attempt to minimize vibrations. See Figure 4.
 |
| Figure 4. CSTM Instrument used by the Hamers Research Group, Department of Chemistry, University of Wisconsin-Madison. On the left are the control electronics used for the STM. The vacuum chamber in which the microscope resides is on the right. The chamber is constructed of stainless steel, which is necessary because of the vacuum under which the microscope is operated (less than 1x10-10 Torr). A closeup of the Vacuum chamber is shown in Figure 5. Photograph by Jack Markham. |
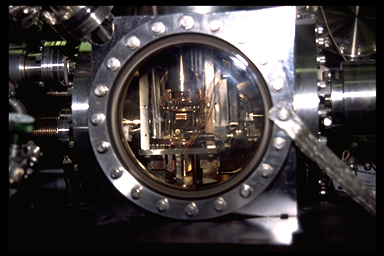 |
| Figure 5. CSTM Vacuum Chamber used by the Hamers Research Group, Department of Chemistry, University of Wisconsin-Madison. Photograph by Jack Markham. |
Often in STM experiments it is necessary to either maintain a clean surface or control the chemicals that come into contact and react with the surface. Many surfaces will react with the gases in the atmosphere. For example, the surface of aluminum exposed to the air is rapidly covered with an oxide Al2O3. Copper also reacts with oxygen to form copper oxides, such as those responsible for the green color of the Statue of Liberty. While the chemistry of metal oxides such as aluminum is important, it is often the surface of the metal itself that we are interested in. It is possible to obtain an STM image of the pure aluminum metal surface only when the sample is kept in an oxygen free environment.
It is sometimes beneficial to examine how one particular molecule reacts with a surface. For example, how does a molecule such as 2,2'-bithiophene interact with a silver surface?
By performing this experiment in the controlled environment of a vacuum chamber (see Figure 5) it is possible to ensure that only bithiophene molecules come into contact with a clean silver surface. You can then observe that the molecules bond only along an atomic step on the surface as shown in Figures 6a and b. Other molecules attach to the silver surface in a similar way, as shown in Figure 6c.
 |
| Figure 6a. STM image of 2,2'-bithiophene molecules attached to a step on a silver surface. |
 |
| Figure 6b. An image of the surface shown in Figure 6a. created with Vistapro. |
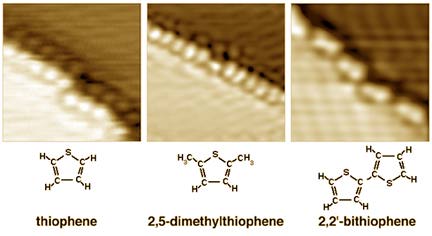 |
| Figure 6c. Additional examples of molecules attached to a silver surface. Note the similarity in attachment at the step. |
This type of study allows scientists to easily control experimental variables. This is necessary to obtain a fundamental understanding of chemistry as it occurs on a surface. As a result, many STM experiments are performed in an extremely clean high vacuum chambers (see figure 5). The pressure inside may be less than 1x10-12 atmospheres, or approximately 1x10-10 Torr.
Most chemical reactions not occur in such a controlled environment such as a vacuum chamber. STM can also be used to watch a chemical reaction or physical change occur on a surface under ordinary conditions, room temperature and atmospheric pressure. For example, dissolution of a solid occurs at the solid surface. Put salt in water, and it is the surfaces of the salt crystals that make contact with the water. By obtaining a series of STM images of the same area of a surface exposed to a solvent, it is possible to make a movie and watch dissolution occur. The movie in Figure 7 shows is the dissolution of a crystal of PbS. Note that material is not removed randomly or uniformly from the surface, but is removed preferentially from steps.
| Figure 7. This QuickTime movie shows the dissolution a lead sulfate crystal. Click the arrow in the control bar to play the movie. |
These two examples demonstrate the real power of STM: by observing surfaces with atomic resolution, chemical and physical differences of small features on surfaces, such as steps, can be studied. While it might make intuitive sense that an atom at the edge of a step will react differently from an atom inside a solid or even within a plane terrace of atoms, it is now possible to observe just how different the chemistry of step atoms is. This has provided a greater understanding of how chemical and physical processes occur on surfaces. STM has helped explain why some surfaces of the same element with different geometric structures react differently, how molecules arrange themselves on surfaces, and even how two different atoms or molecules on a surface interact with each other. This has led to advances in the ability to control the chemistry at the atomic scale, making possible the production of microscale machines and electronic components.
Regardless of how or where STM experiments are performed, the resolution with which chemical and physical processes are observed has provided new and breathtaking insights on what happens on the surfaces of materials. The examples used here show just a few such studies. More information is available in the scientific literature.
How to Interpret STM Images
While it is often said that an STM shows atoms, in the strictest sense this is not completely true. Tunneling electrons are used to generate the image and only areas where there are electrons will generate a tunneling current. Since the highest electron density is usually located around the nucleus of an atom, the probability of electron tunneling is highest near the atomic nucleus. The tunneling current is highest near the nucleus. This area appears as a protrusion in an STM image. If electrons tunnel from the sample to the tip, the STM image looks at filled electronic states of the sample. In other words, a filled states STM image records areas of high electron density of the sample. If electrons tunnel from the tip to the sample surface, the electrons must go into areas where electrons can reside but that are currently empty. These are called empty state images of the sample. In other words, the STM "sees" the location of or possible location of electrons. Since most electrons are located near the nucleus of an atom, the position of atoms can be determined.
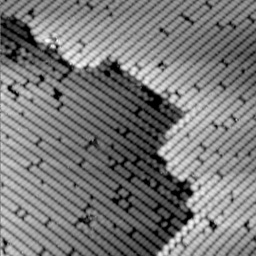
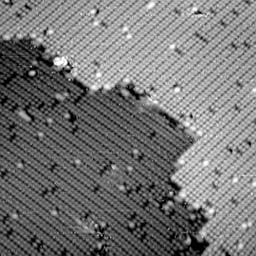
However, electrons are often located some distance away from the nucleus of an atom. When a chemical bond forms between two atoms, there is a higher electron density between the two nuclei. Silicon, for example, forms chemical bonds similar to carbon. On the silicon (100) crystal surface (the (100) simply denotes a plane along the crystal face. See Figure 8 for the image of Si (111)), adjacent silicon atoms form a weak p-type bond which has a high electron density distributed between to two atoms. Consequently, when a "filled" states image of this surface is acquired, the probability of having an electron tunnel will be higher between the two silicon nuclei and the protrusion in the STM image will appear between the atomic nuclei. The resulting bean shape (see Figure 9a) is observed in this STM image is a result of the weak chemical bond formed between two silicon atoms at the surface. Conversely, when an "empty" states image of this same silicon surface is obtained, a node, or depression, appears where the "beans" were observed in the filled states image (see Figure 9b). This is because the only empty spaces for tunneling electrons to go are away from the area between the two silicon atoms.
 |
|
Figure 8. Si(111) 7X7 Image of a clean silicon surface. The crystal plane image is the (111) not the (100) surface in the other images (Figures 9a and 9b). The difference in appearance is due to the different atomic positions on these two crystal faces. |
 |
|
 |
|
Figure 9 a. A filled state image of a silicon (100) surface. |
|
Figure 9b. An empty state image of the same surface. |
 |
|
Figure 10. STM image of the surface of silver. No protrusions marking the site of atomic nuclei are visible. |
Compare Figures 8, 9a, and 9b with Figure 10, an STM image of the surface of silver. The silicon and silver images look very different. In metal solids, such as silver, the electrons are delocalized. Electron density is fairly uniform across the surface. As a result, protrusions caused by the slightly higher electron density around atomic nuclei are much more difficult to observe. In the silver image shown here, the silver atomic nuclei positions are not imaged, and the only structural features observed in the STM images are to geometric changes in height such as steps.
|
|
|
Copyright © 1998 Division of Chemical Education Inc. of the American Chemical Society. All rights reserved. |