|
Home
Table of Contents
Keyword Index
Textbook X-ref
Search
About CCA!
|
 |
| |
Click a thumbnail picture below to view a larger version. |

View Movie |
|
Narrative
Solid potassium permanganate is added to benzene. The ionic solid does not dissolve in the nonpolar organic liquid. When the crown ether 18-dibenzo-crown-6 is added, the potassium permanganate dissolves forming a purple solution. The crown ether forms a host-guest complex with potassium ion, and the resulting complex is soluble in benzene.
Discussion
Crown ethers were discovered by Charles Peterson who shared the Nobel Prize in Chemistry in 1987 with J. M. Lehn and Donald Cram for developments in the field of host-guest complexes.
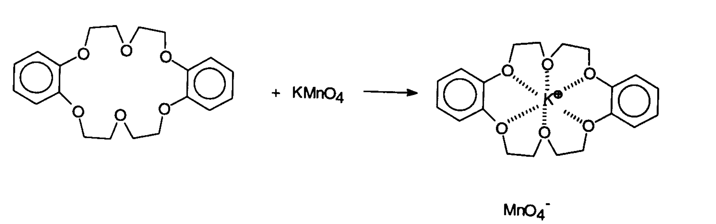
...with the MNO4- soluble in benzene |
|
|
|
|
Molecular model of 18-dibenzo-crown-6 |
|
|
|
|
|

