TOPIC: 3. Alkanes / Cycloalkanes
SUBTOPIC: 32. Conformational Analysis
1. Draw the rotational conformers of 2-methylbutane about the 2,3 bond in Newman projection at 60o intervals. Rank them in order of stability. The most stable is 1.
____________________________________________________________
ANS:


____________________________________________________________
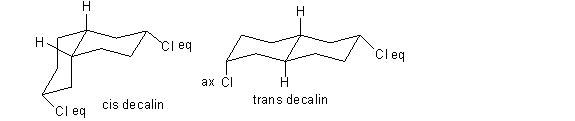
2. Two decalin isomers are shown in the box below. Complete the chair conformation drawings by adding the hydrogens at the ring fusions and the chloro substituents in the proper orientation. Label the chloro substituents as axial or equatorial. Label the chair conformations as cis decalin or trans decalin.

____________________________________________________________
ANS:
____________________________________________________________
3. Draw the anti and gauche staggered conformations of 1,2-difluoroethane using wedge and dash 3-D structures. Label them as anti or gauche. Estimate the dipole moment for each.
____________________________________________________________
ANS:

____________________________________________________________
4. Convert the following 3-dimensional wedge and dash drawing into the identical Newman projection.

____________________________________________________________
ANS:

____________________________________________________________
5. Draw the two ring flip conformations of cis,trans-1,3,5-trimethylcyclohexane. Circle the most stable one.
Determine the difference in energy between the two ring flip conformations (ΔGo).
Calculate the
ratio of the two conformations at room temperature (25o C). A
methyl–hydrogen 1,3 diaxial interaction is worth 3.8
kJmol-1 and a methyl-methyl 1,3 diaxial
interaction is 15.4 kJmol-1.
ΔGo
= -2.303RT log Keq, R = 8.314 J K-1
__________________________________________________________________________________________
ANS:

________________________________________________________
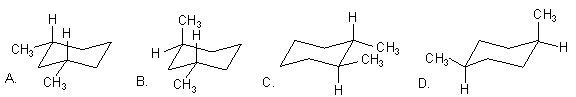
6. Which is the most stable cyclohexane?
____________________________________________________________
ANS: A.
____________________________________________________________
7. Draw the gauche conformation of 2-chloroethanol in a Newman projection.
____________________________________________________________
ANS:

___________________________________________________________
8. Draw the three possible staggered conformations of 2,3-dimethylbutane about the C2 – C3 bond in Newman projection. Rank them in order of stability (most stable first). Write the number of gauche interactions under each structure.
____________________________________________________________
ANS:

____________________________________________________________
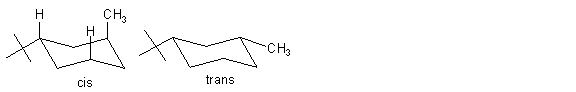
9. Draw the most stable chair conformation of cis-1-tert-butyl-3-methylcyclohexane and trans-1-tert-butyl-3-methylcyclohexane. Which isomer is more stable, cis or trans? Why?
____________________________________________________________
ANS: The trans isomer is more stable because both groups are equatorial. The cis isomer has an axial methyl which has 2 steric interactions with the axial hydrogens on the same face.
____________________________________________________________
10. β-Glucopyranose is a six-membered ring in which all the substituents are equatorial. Draw β-glucopyranose in its most stable chair conformation. Put in all the hydrogens.

____________________________________________________________
ANS:

____________________________________________________________
11. Draw the most stable conformation of 3,4-dimethylhexane in Newman projection about the 3,4 bond.

____________________________________________________________
ANS:

____________________________________________________________
12. Convert the following Newman projection into the identical wedge and dash 3-D drawing.

____________________________________________________________
ANS:

____________________________________________________________
13. Convert the following Newman projection into the identical wedge and dash 3-D drawing.

____________________________________________________________
ANS:

____________________________________________________________
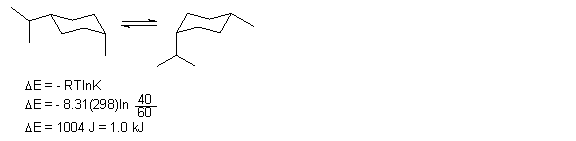
14. Draw the two ring flip chair conformations of cis-1-isopropyl-4-methylcyclohexane. If the more stable conformation comprises 60% of the equilibrium mixture at 25o C, what is the energy change for the ring flip. ΔE = - RTln K, R = 8.31 J/Kmol
____________________________________________________________
ANS:
____________________________________________________________
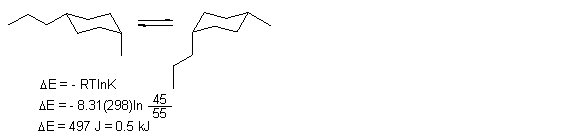
15. Draw the two ring flip chair conformations of cis-1-methyl-4-propylcyclohexane. If the more stable conformation comprises 55% of the equilibrium mixture at 25o C, what is the energy change for the ring flip. ΔE = - RTln K, R = 8.31 J/Kmol
____________________________________________________________
ANS:
____________________________________________________________