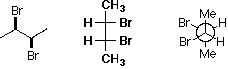
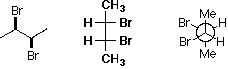
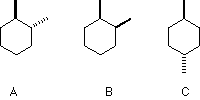
Choose the thermodynamically least stable isomer.
A B C
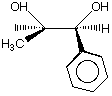 has how many stereo centers? 0, 1, 2
has how many stereo centers? 0, 1, 2
 , can be made this way.
, can be made this way.
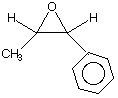
How many enantiomers of phenylpropylene oxide are there? 1, 2, 4
1049. Choose the correct products for these reactions:
a.
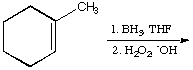
A. 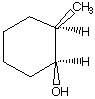 B.
B. 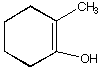 C.
C. 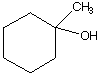 D.
D. 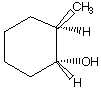
b.
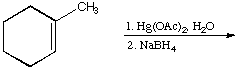
A.  B.
B.  C.
C.  D.
D. 
a.
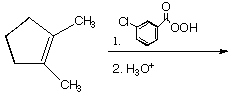
A. 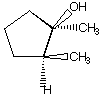 B.
B. 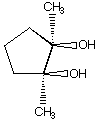 C.
C. 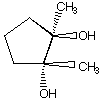 D.
D. 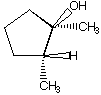
b.
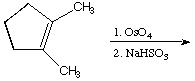
A.  B.
B.  C.
C.  D.
D. 
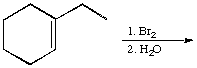
A. 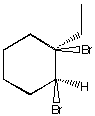 B.
B. 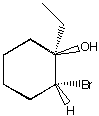 C.
C. 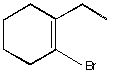
D. 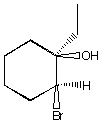
1084. a. What is the R and S configuration for each stereogenic center in this sugar, from top to bottom?
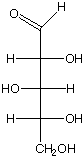
R, R, S R, S, S R, S, R
b. Is this a D or L sugar?
c. What is the R and S configuration for each stereogenic center in this sugar, from top to bottom?
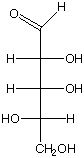
R, R, S R, S, S R, S, R
b. Is this a D or L sugar?
1095. How many isomers have the name bromomethylcyclopentane? (ignoring chirality)
A. 4
B. 5
C. 6
D. 7
1097. How many stereocenters (not including any N centers) are there in morphine, whose structure is shown below?
A. 4
B. 5
C. 6
D. More than 6
1098. Is the structure shown below R or S?
A. R
B. S
1099. Is the structure shown below R or S?
A. R
B. S
1100. The stereochemistry of this molecule is:
A. 1R, 3R
B. 1R, 3S
C. 1S, 3S
D. 1S, 3R
1101. Pure (S)-2-butanol has a specific rotation of +13.52 degrees. A sample of 2-butanol prepared in the lab and purified by distillation has a calculated specific rotation of +6.76 degrees. What can you conclude about the composition?
A. 50% (S), 50% impurity
B. 50% (S), 50% (R)
C. 50% (S), 50% racemic
D. some other mixture
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..