![]()
Compared to lighter atoms at the same temperature, heavier atoms on average
move faster, move slower, move at the same average velocity
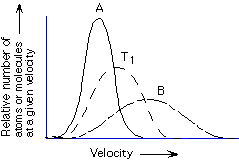
A, B, T1
best for a dilute gas, best for a concentrated gas
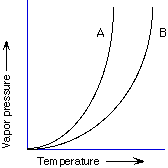
A, B
Demonstration: Syringes are used to bring drops of H2O
and ethanol to the top of columns of Hg, as shown below. What will happen
to the heights of the Hg columns?
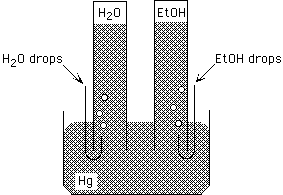
both column heights will be the same, the column below H2O is shorter, the column below EtOH is shorter
>100 °C, 100 °C, <100 °C
higher temperature with CaCl2, lower temperature with CaCl2, no difference
pure H2O, H2O and salt, pure salt
number of gas moles: goes up, goes down, stays constant
temperature: goes up, goes down, stays constant
volume of trapped gas: goes up, goes down, stays constant
pressure of trapped gas: goes up, goes down, stays constant
number of gas moles: goes up, goes down, stays constant
temperature: goes up, goes down, stays constant
volume of trapped gas: goes up, goes down, stays constant
pressure of trapped gas: goes up, goes down, stays constant
What will happen to the egg?
nothing, it will pop out of the flask, it will be sucked into the flask
number of gas moles: goes up, goes down, stays constant
temperature: goes up, goes down, stays constant
volume of trapped gas: goes up, goes down, stays constant
pressure of trapped gas: goes up, goes down, stays constant
zero, one, many
The layer sequence of LiCl is shown below. The reaction for dissolving
the extended solid in water is LiCl (s) --> Li+ (aq) + Cl- (aq).
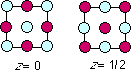
How many bonds are broken per LiCl formula unit when a chunk of LiCl dissolves?
zero, one, many
left end, right end
Will a soap solution be an electrolyte? Demonstration: Show that
a light bulb or LED with exposed leads lights up when the leads are immersed
in soapy water.
yes, no
Demonstration: Gently sprinkle lycopodium powder on top of a Petri
dish of water placed on an overhead projector. Take a toothpick, stick it
into a bar of soap, and then poke the toothpick into the center of the vessel.
The particles will be pushed to the edge by the soap molecules. Alternately,
a paper clip can be shown to float and then sink when soap is added.
How will the anions on the surface of a beaker of water prefer to arrange
themselves:
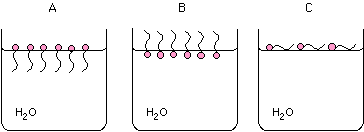
A, B, C
How does soap interact with grease (see below)?
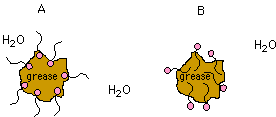
A, B
A, B, C
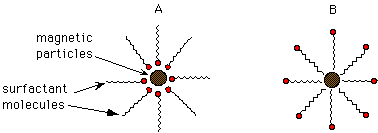
A, B
the symmetric high temperature phase, the less symmetric low temperature phase
To cause the ring-sounding sample to give a thudding sound,
heat it, cool it
Demonstration: cool the sample with liquid nitrogen, remove it
and drop it to hear a thud. Rapid hand warming will eventually restore the
ring during repeated drops.
Which phase is more mechanically flexible?
the symmetric high temperature phase, the less symmetric low temperature phase
When nickel titanium memory metal interconverts between the symmetric high
temperature form and the less symmetric low temperature form, which of the
following changes?
elemental analysis, X-ray diffraction pattern, hardness
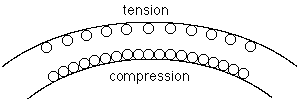
When a sample of nickel-titanium in the high temperature phase is bent,
as pictured, the atoms that are under compression and thus favored by Le
Châtelier's principle to convert to the denser low-temperature phase
are those
at the bottom of the bend, in the middle of the bend, at the top of the bend
Demonstration 9.5 "Companion": Bend memory metal eyeglass frames and show that they return to their original shape. Then cool with liquid nitrogen to show that when the eyeglass frames are in the more flexible low temperature phase they stay bent until they return to room temperature, where they regain their original shape.
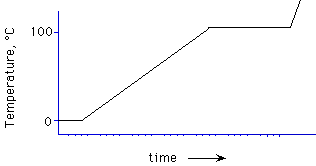
ice melting, water boiling
the color of the vapor in that tube will become darker, there is no change in the color of the vapor, the color of the vapor in that tube will become lighter
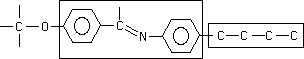
13, 17, 21
Which part of the molecule is more rigid?
left box, right box
Schematically, the solid liquid crystal has molecules aligned in a repeating pattern. In the liquid, order is lost. In the liquid crystal, there is still orientational order. Phase transitions are observed at 21 and 45 degrees C.
In which region would the liquid crystal be the stable phase?
A, B, C
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..